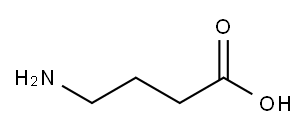
4-Aminobutyric acid synthesis
- Product Name:4-Aminobutyric acid
- CAS Number:56-12-2
- Molecular formula:C4H9NO2
- Molecular Weight:103.12

21994-40-1
5 suppliers
inquiry

56-12-2
890 suppliers
$5.00/100mg
Yield:-
Reaction Conditions:
Stage #1: 4-azidobutanenitrilewith trisodium thiophosphate in water-d2 at 90; for 3 h;
Stage #2: with [D]-sodium hydroxide in water-d2 at 90; for 16 h;
Steps:
Experimental procedures for entries in Table 2
Entry 8: Trisodium thiophosphate (140 μmol, 25 mg) was dissolved in D2O (1 mL) and the solution was added to 4-azido-butyronitrile (45 μmol, 5 mg) in an NMR tube. The NMR tube was heated at 90 °C for 3 h, and allowed to cool. 100 μl of 40% sodium deuteroxide in deuterium oxide was added to the NMR tube, and was heated at 90 °C for 3 h before being subjected to 1H, 13C NMR analyses. To confirm the formation of γ-aminobutyric acid, commercial GABA was added to the NMR tube, with 1H and 13C NMR analyses being performed after addition.
References:
Norcliffe, Jennifer L.;Conway, Louis P.;Hodgson, David R.W. [Tetrahedron Letters,2011,vol. 52,# 21,p. 2730 - 2732] Location in patent:supporting information; body text
![Spiro[2,4-benzodioxepin-3,1'-cyclopropane]-2'-carbonitrile, 1,5-dihydro-](/CAS/20210305/GIF/100340-10-1.gif)
100340-10-1
0 suppliers
inquiry

56-12-2
890 suppliers
$5.00/100mg

56-86-0
838 suppliers
$5.00/25g

56-12-2
890 suppliers
$5.00/100mg
![(S)-4-[(Allyloxycarbonyl)amino]butanoic acid](/CAS/20200331/GIF/80127-29-3.gif)
80127-29-3
2 suppliers
inquiry

56-12-2
890 suppliers
$5.00/100mg
