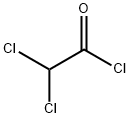
Dichloroacetyl chloride synthesis
- Product Name:Dichloroacetyl chloride
- CAS Number:79-36-7
- Molecular formula:C2HCl3O
- Molecular Weight:147.39
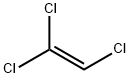
79-01-6
438 suppliers
$19.21/100ml

79-36-7
284 suppliers
$15.00/10g
Yield:79-36-7 90%
Reaction Conditions:
with tributyl borane;oxygen at 80; under 2280.15 Torr; for 11 h;Reagent/catalyst;
Steps:
1.1; 2.1; 3.1
Add 1314g (10mol) of trichloroethylene and 18.2g (0.1mol) of tributylboron into the pressure-resistant reactor, then pass in dry oxygen, adjust the pressure of the oxygen to 3 atmospheres, and then increase the temperature React at 80°C for 11h. After the completion of the reaction, atmospheric distillation is performed, and the fractions are collected at a temperature range of 105°C to 108°C to obtain 1,325 g of a colorless and transparent liquid product, dichloroacetyl chloride, with a yield of 90%.
References:
CN111995516,2020,A Location in patent:Paragraph 0041; 0043-0044; 0058; 0060-0061; 0066; 0068-0069

79-43-6
292 suppliers
$22.00/25g

79-36-7
284 suppliers
$15.00/10g

75-36-5
563 suppliers
$17.92/100G

79-36-7
284 suppliers
$15.00/10g

79-04-9
354 suppliers
$12.00/5g
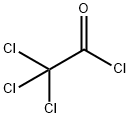
76-02-8
251 suppliers
$38.29/25G

76-02-8
251 suppliers
$38.29/25G

79-36-7
284 suppliers
$15.00/10g
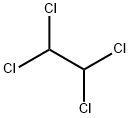
79-34-5
245 suppliers
$10.00/5g

79-36-7
284 suppliers
$15.00/10g
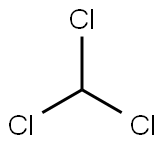
67-66-3
6 suppliers
$33.60/500ml
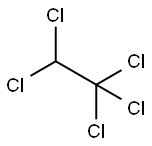
76-01-7
56 suppliers
$57.20/5ml