
4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone synthesis
- Product Name:4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone
- CAS Number:80912-60-3
- Molecular formula:C13H14O3
- Molecular Weight:218.25
![1,4-Dioxaspiro[4.5]decane, 8-(1,3-benzodioxol-5-yl)-](/CAS/20210305/GIF/82749-42-6.gif)
82749-42-6
0 suppliers
inquiry
![4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone](/CAS/20150408/GIF/80912-60-3.gif)
80912-60-3
6 suppliers
inquiry
Yield:80912-60-3 98%
Reaction Conditions:
with hydrogenchloride in acetone at 20; for 4 h;
Steps:
N-{[1-(4-Benzo[1,3]dioxol-5-yl-cyclohexyl)-azetidin-3-ylcarbamoyl]-methyl}-3-trifluoromethyl-benzamide (8a and 9a)
General procedure: N-(Azetidin-3-ylcarbamoylmethyl)-3-trifluoromethyl-benzamide TFA salt: 3-Amino-azetidine-1-carboxylic acid tert-butyl ester (1.2 g, 6.97 mmol) and (3-trifluoromethyl-benzoylamino)-acetic acid (1.57 g, 6.36 mmol) were treated with EDCI (1.57 g, 6.36 mmol), HOBT (1.22 g, 6.36 mmol) in DCM (10 mL) at room temperature for 4 hours. The reaction solution was partitioned between DCM and water. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated to give yellow oil, which was then purified by silica gel column on a CombiFlash system using hexanes and ethyl acetate (from 10% ethyl acetate to 100% ethyl acetate) to afford 3-[2-(3-trifluoro-methyl-benzoylamino)-acetylamino]-azetidine-1-carboxylic acid tert-butyl ester as white solid, 2.23 g, 80% yield. 1H NMR (400 MHz, CDCl3) d 8.10 (s, 1H), 8.02 (d, J = 6.6 Hz, 1H), 7.80 (d, J = 6.8 Hz, 1H), 7.56 (t, J = 6.5 Hz, 1H), 4.61 (m, 1H), 4.25 (t, J = 7.2 Hz, 2H), 4.18 (d, J = 5.5 Hz, 2H), 3.82 (t, J = 7.5 Hz, 2H), 1.41 (s, 9H). 3-[2-(3-Trifluoromethyl-benzoylamino)-acetylamino]-azetidine-1-carboxylic acid tert-butyl ester (2.10 g, 5.24 mmol) was dissolved in 1:1 TFA and DCM mixed solution (10 mL) at room temperature. The reaction was stirred for another 2 hours. The solvent was removed and the residue was dried to give N-(azetidin-3-ylcarbamoylmethyl)-3-trifluoromethyl-benzamide as a TFA salt containing extra TFA (colorless oil), ~ 2.5 g, 100% yield. 1H NMR (400 MHz, CDCl3) d 8.10 (s, 1H), 8.05 (d, J = 6.0 Hz, 1H), 7.78 (d, J = 6.2 Hz, 1H), 7.55 (m, 2H), 4.78 (m, 1H), 4.15 (d, J = 3.2 Hz, 2H), 3.95 (t, J = 7.0 Hz, 2H), 3.52 (t, J = 7.0 Hz, 2H).8-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-1,4-dioxa-spiro[4.5]dec-7-ene (PCT Int. Appl. WO2006064189, 0.292 g, 1.10 mmol), 5-bromobenzo[d][1,3]dioxole (Aldrich, 161 mg, 0.801 mmol), and tetrakis (triphenylphosphino)palladium(0) (Aldrich, 0.048 g, 0.042 mmol) were dissolved in 1,4-dioxane (9 mL), treated with 2M aqueous Na2CO3 (2.0 mL, 4.0 mmol), bubbled with argon for a few minutes, and heated to 100oC under reflux condenser for 24 h. After cooling to ambient temperature, the reaction was diluted with water (30 mL), extracted thrice with dichloromethane, aqueous layer acidified to ca. pH 7, extracted twice more with dichloromethane, and the combined organic layers washed with brine, dried over Na2SO4, filtered, and the filtrate concentrated in vacuo to give an orange oil. This was purified by thin layer chromatography on silica gel (EtOAc) to give 5-(1,4-dioxaspiro[4.5]dec-7-en-8-yl)benzo[d][1,3]dioxole as a yellow solid (110 mg, 53%). 5-(1,4-dioxaspiro[4.5]dec-7-en-8-yl)benzo[d][1,3]dioxole (110 mg, 0.42 mmol) in MeOH (5 mL) was placed in a hydrogenation Par Shaker under 40 psi at room temperature using 5% Pd/C (~ 50 mg) as catalyst for 2 hour. The resulting solution was filtered through a pad of Celite , concentrated and purified by silica gel column on a CombiFlash system to afford 5-(1,4-dioxaspiro[4.5]decan-8-yl)benzo[d][1,3]dioxole as white solid, 91 mg, 82% yield. 5-(1,4-dioxaspiro[4.5]decan-8-yl)benzo[d][1,3]dioxole (91 mg, 0.35 mmol) was treated with 1N HCl ( ~ 2 mL) in acetone (4 mL) at room temperature for 4 hours. The reaction was neutralized with saturate NaHCO3 solution and the solvent was removed. The residue was partitioned between ethyl acetate and water. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated to give yellow solid, which was then purified by silica gel column on a CombiFlash system using hexanes and ethyl acetate (from 10% ethyl acetate to 100% ethyl acetate) to afford 4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone as white solid, 75 mg, 98% yield.4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone (75 mg, 0.35 mmol) and N-(azetidin-3-ylcarbamoylmethyl)-3-trifluoromethyl-benzamide TFA salt (151 mg, 0.50 mmol) in DCM (2 mL) was treated with TEA (0.1 mL, 0.75 mmol) for 10 min followed by NaBH(OAc)3 (Aldrich, 225 mg, 1.05 mmol) for another 4 hours at room temperature. The reaction was quenched with saturated sodium bicarbonate. The organic layer was separated and the aqueous layer was extracted 3 times with chloroform and IPA "cocktail" (~ 3:1, v/v). The combined organic layer was dried over anhydrous Na2SO4, filtered and concentrated to give the crude product, which was then purified by a CombiFlash system using ethyl acetate and 7N NH3 in MeOH as eluent (from pure ethyl acetate to 5% 7N NH3 in MeOH in ethyl acetate) to afford two title compounds as white solids: 8a, less polar isomer (cis), 67 mg, 38% yield; 9a, more polar isomer (trans), 45 mg, 26% yield.
References:
Zhang, Xuqing;Hufnagel, Heather;Markotan, Thomas;Lanter, James;Cai, Chaozhong;Hou, Cuifen;Singer, Monica;Opas, Evan;McKenney, Sandra;Crysler, Carl;Johnson, Dana;Sui, Zhihua [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 18,p. 5577 - 5582] Location in patent:supporting information; experimental part
![4-(BENZO[D][1,3]DIOXOL-5-YL)CYCLOHEX-3-ENONE](/CAS/20180601/GIF/1252615-58-9.gif)
1252615-58-9
5 suppliers
inquiry
![4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone](/CAS/20150408/GIF/80912-60-3.gif)
80912-60-3
6 suppliers
inquiry
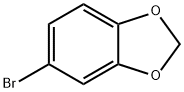
2635-13-4
299 suppliers
$5.00/1g
![4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone](/CAS/20150408/GIF/80912-60-3.gif)
80912-60-3
6 suppliers
inquiry
![1,4-Dioxaspiro[4.5]dec-7-ene, 8-(1,3-benzodioxol-5-yl)-](/CAS/20210305/GIF/114046-96-7.gif)
114046-96-7
0 suppliers
inquiry
![4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone](/CAS/20150408/GIF/80912-60-3.gif)
80912-60-3
6 suppliers
inquiry
![8-(benzo[d][1,3]dioxol-5-yl)-1,4-dioxaspiro[4.5]decan-8-ol](/CAS/20150408/GIF/150019-56-0.gif)
150019-56-0
8 suppliers
inquiry
![4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone](/CAS/20150408/GIF/80912-60-3.gif)
80912-60-3
6 suppliers
inquiry