
4-Benzoxazolamine,2-methyl-(9CI) synthesis
- Product Name:4-Benzoxazolamine,2-methyl-(9CI)
- CAS Number:342897-54-5
- Molecular formula:C8H8N2O
- Molecular Weight:148.16

478553-83-2
20 suppliers
$128.00/500mg

342897-54-5
33 suppliers
$45.00/10mg
Yield:342897-54-5 100%
Reaction Conditions:
with iron;ammonium chloride in tetrahydrofuran;ethanol;water at 70; for 2 h;
Steps:
173.2 Step 2
2-Methylbenzo[d]oxazol-4-amine (Compound 173-2)
Compound 173-1 (11.0 g, 61.79 mmol) was dissolved in THF (50 mL), ethanol (50 mL), and water (50 mL), and iron powder (17.30 g, 308.99 mmol) and ammonium chloride (4.91 g, 92.70 mmol) were added to the solution at room temperature.
The mixture was stirred at 70°C for 2 hours.
The mixture was cooled to room temperature, and concentrated under reduced pressure.
The residue/organic layer was extracted with ethyl acetate, washed with saturated saline, dried over anhydrous sodium sulfate, and concentrated under reduced pressure.
The residue was crystallized with n-pentane, and compound 173-2 (9.2 g, quantitatively) was obtained by filtering the crystals obtained.
1H NMR (400 MHz, CDCl3, δ): 7.51 (d, J = 8.4 Hz, 1H), 6.62 (d, J = 8.4 Hz, 1H), 5.99 (s, 1H), 4.31 (brs, 2H), 3.86 (s, 3H);
ESIMS m/z: [M + H]+ 149.
References:
EP3712129,2020,A1 Location in patent:Paragraph 1326

478553-83-2
20 suppliers
$128.00/500mg

7439-89-6
423 suppliers
$10.00/25g

342897-54-5
33 suppliers
$45.00/10mg

603-85-0
260 suppliers
$15.00/5g

342897-54-5
33 suppliers
$45.00/10mg
![Acetamide, N-[2-(acetyloxy)-6-nitrophenyl]-](/CAS/20210305/GIF/69194-51-0.gif)
69194-51-0
0 suppliers
inquiry

342897-54-5
33 suppliers
$45.00/10mg
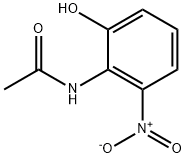
59820-29-0
0 suppliers
inquiry

342897-54-5
33 suppliers
$45.00/10mg