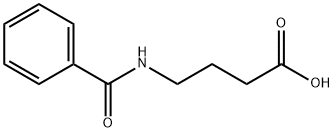
4-(Benzoylamino)butyric acid synthesis
- Product Name:4-(Benzoylamino)butyric acid
- CAS Number:35340-63-7
- Molecular formula:C11H13NO3
- Molecular Weight:207.23

98-88-4
577 suppliers
$10.00/5g
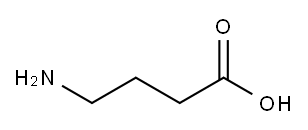
56-12-2
843 suppliers
$5.00/100mg

35340-63-7
32 suppliers
$44.00/100mg
Yield:35340-63-7 94%
Reaction Conditions:
with sodium hydroxide in water at 0 - 20;Inert atmosphere;
Steps:
4-Benzamido butanoic acid
γ-Aminobutyric acid (0.30 g, 2.91 mmol) and NaOH (0.21 g, 5.24 mmol) were dissolved in H20 (6 mL). The resulting solution was cooled to 0 °C and to this was added dropwise benzoyl chloride (0.31 mL, 2.63 mL). The mixture was stirred for two hours at 0 °C and then at room temperature overnight. The resulting solution was acidified with cone. HC1 to pH 2, extracted with ethyl acetate (3 x 30mL), dried over Na2S04, and concentrated in vacuo. The residue was taken up in minimal ethanol followed by the addition of water until a cloudy solution was formed. The solution was concentrated to yield a white solid which was triturated with hexane and filtered to yield a crystalline solid (1.41 g, 94%); mp 88-89 °C. lR NMR (500 MHz, methanol-^) 5 7.79-7.87 (m, J=0.95, 8.51 Hz, 1H), 7.50-7.56 (m, 2H), 7.43-7.50 (m, 3H), 3.45 (t, j=6.94 Hz, 2H), 2.41 (t, J=7.25 Hz, 2H), 1.94 (quin, J=7.17 Hz, 2H). 13C MR (126 MHz, methanol-^) δ 182.1, 137.5, 136.9, 129.4, 128.3, 42.5, 36.1, 24.8, 21.3.
References:
WO2014/18913,2014,A2 Location in patent:Page/Page column 46
3389-54-6
25 suppliers
inquiry
2399-66-8
38 suppliers
$100.00/1g
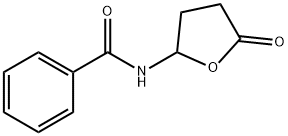
36566-48-0
0 suppliers
inquiry

35340-63-7
32 suppliers
$44.00/100mg

3389-54-6
25 suppliers
inquiry

35340-63-7
32 suppliers
$44.00/100mg

3389-54-6
25 suppliers
inquiry

2399-66-8
38 suppliers
$100.00/1g

35340-63-7
32 suppliers
$44.00/100mg
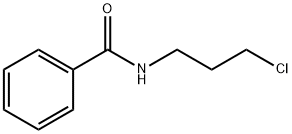
10554-29-7
1 suppliers
inquiry

35340-63-7
32 suppliers
$44.00/100mg