
4-BENZYL-5-(2-THIENYL)-1,2,4-TRIAZOLE-3-THIOL synthesis
- Product Name:4-BENZYL-5-(2-THIENYL)-1,2,4-TRIAZOLE-3-THIOL
- CAS Number:139614-67-8
- Molecular formula:C13H11N3S2
- Molecular Weight:273.38

139614-66-7
5 suppliers
inquiry

139614-67-8
10 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium hydroxide for 6 h;Reflux;
Steps:
4-Benzyl-5-(thiophene-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (1).
A mixture of thiophene-2-carbohydrazide (I) (0.01 mol), ethanol (50 mL), andbenzyl isothiocyanate was refluxed for 3 h. After ~4 h,solid N-benzyl-2-(thiophene-2-carbonyl)hydrazine-1-carbothioamide began to separate from the solution.Potassium hydroxide (0.15 mol) was then added, andthe mixture was refluxed for 6 h, cooled, acidified topH 3-4 with aqueous HCl, and poured onto crushedice while stirring. The resulting solid was filtered off,dried, and recrystallized from ethyl alcohol. Yield 65%,mp 145-147°C. IR spectrum (KBr), ν, cm-1: 3064-3102 (C-Harom), 2854, 2967 (C-H), 1574 (C=N), 1274(C=S), 705 (C-S-C). 1H NMR spectrum (400 MHz,DMSO-d6), δ, ppm: 5.48 s (2H, CH2C6H5), 7.10-7.74 (8H, Harom), 14.16 s (1H, SH). 13C NMR spectrum(100 MHz, DMSO-d6), δC, ppm: 47.2, 126.6, 127.9,129.1, 129.3, 130.3, 135.8, 146.7.0, 168.2. Found, %:C 57.02; H 4.15; N 15.07; S 23.21. C13H11N3S2.Calculated, %: C 57.12; H 4.06; N 15.37; S 23.46.
References:
Sarac;Orek;Koparir [Russian Journal of Organic Chemistry,2021,vol. 57,# 1,p. 100 - 107][Zh. Org. Khim.,2021,vol. 57,# 1,p. 100 - 107,8]
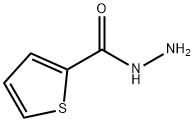
2361-27-5
217 suppliers
$10.00/1g

139614-67-8
10 suppliers
inquiry
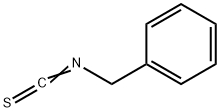
622-78-6
237 suppliers
$12.00/1g

139614-67-8
10 suppliers
inquiry