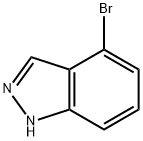
4-Bromo-1H-indazole synthesis
- Product Name:4-Bromo-1H-indazole
- CAS Number:186407-74-9
- Molecular formula:C7H5BrN2
- Molecular Weight:197.03
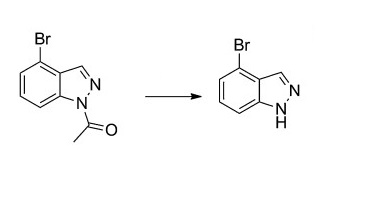
55289-36-6
280 suppliers
$5.00/1g

186407-74-9
310 suppliers
$5.00/1g
Yield:186407-74-9 95%
Reaction Conditions:
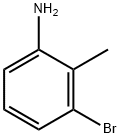
Stage #1: 3-bromo-2-methylanilinewith acetic anhydride in chloroform at 20; for 1 h;
Stage #2: with potassium acetate;isopentyl nitrite for 18 h;Heating / reflux;
Stage #3: with hydrogenchloride at 50; for 2 h;Heating / reflux;
Steps:
71
Acetic anhydride (2.27 eqiv) was added to a cooled 0 C solution of bromomethylaniline (1.00 eqiv) in chloroform (1.5 mL/mol) while maintaining the temperature below 40 C. The reaction mixture was allowed to warm to room temperature and was maintained for 1 h. Potassium acetate (0.29 eq) and isoamyl nitrite (2.15 eqiv) was added and the reaction mixture was heated at reflux for 18 h. The volatiles were removed under reduced pressure. Water (0.65 L/mol) was added to the residue and the mixture was concentrated. Concentrated hydrochloric acid (1 L/mol) was added to the residue and the mixture was heated at 50 C for 2 h. The mixture was allowed to cool to room temperature and the pH was adjusted to 10 by the slow addition of a 50% aqueous sodium hydroxide solution. The mixture was diluted with water (0.65 L/mol) and was extracted with ethyl acetate (2 x 1.2 L/mol). The combined extracts were washed with brine (1 L/mol) and dried over anhydrous sodium sulfate. The organic solution was filtered through a plug of silica gel (ethyl acetate wash), concentrated, and the residue was triturated with heptane (1 L/mol). The solids were collected by filtration, rinsed with heptane, and dried in a vacuum oven.
References:
WO2004/29050,2004,A1 Location in patent:Page 62;63

885698-70-4
36 suppliers
$115.00/500mg

186407-74-9
310 suppliers
$5.00/1g

55289-36-6
280 suppliers
$5.00/1g
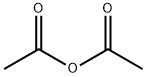
108-24-7
5 suppliers
$14.00/250ML

885698-70-4
36 suppliers
$115.00/500mg

186407-74-9
310 suppliers
$5.00/1g

55289-36-6
280 suppliers
$5.00/1g

108-24-7
5 suppliers
$14.00/250ML

186407-74-9
310 suppliers
$5.00/1g
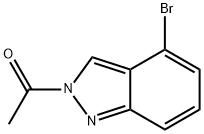
870526-64-0
1 suppliers
inquiry

186407-74-9
310 suppliers
$5.00/1g