4-BROMO-2-(CARBOXYMETHYL)BENZOIC ACID synthesis
- Product Name:4-BROMO-2-(CARBOXYMETHYL)BENZOIC ACID
- CAS Number:943749-63-1
- Molecular formula:C9H7BrO4
- Molecular Weight:259.05
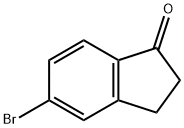
34598-49-7
366 suppliers
$14.00/5g

943749-63-1
27 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:5-Bromo-1-indanone with sodium methylate;oxalic acid diethyl ester in toluene at 0 - 20; for 1.5 h;
Stage #2: with potassium hydroxide;dihydrogen peroxide in methanol;water at 20 - 64; for 17 h;
Stage #3: with hydrogenchloride in methanol;tert-butyl methyl ether;water
Steps:
17.i
A solution of 5-bromo-1-indanone (25 g) and diethyl oxalate (27.7 g) in toluene (500 ml_) was added to a suspension of sodium methoxide (13.1 g) in toluene (50 ml_) at O0C. The mixture was warmed to room temperature and stirred for 90 minutes. The solvent was removed and the residue suspended in methanol (80OmL). Potassium hydroxide (53.1 g) was added portionwise keeping the temp below 500C. Hydrogen peroxide solution (30% w/w, 135 ml_) was then added keeping the temperature below 640C, the reaction mixture was then stirred at room temperature for 17 hours. Sodium sulfite (5% aqueous solution) was added and the reaction stirred for 30 minutes. The reaction mixture was filtered and the filtrate was reduced to half volume then washed with 2-methoxy-2-methyl-propane. The aqueous layer was then acidified with hydrochloric acid and extracted into ethyl acetate. The organic phase was dried, filtered and evaporated then triturated with diethyl ether to yield sub-title compound (9.Og).1H NMR DMSOd6 7.82 (1H, d), 7.62 (1H, d), 7.59 (1H, dd), 3.95 (2H, s).
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2008/122765, 2008, A1 Location in patent:Page/Page column 101
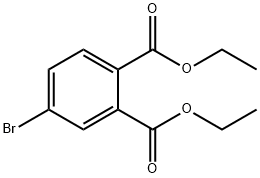
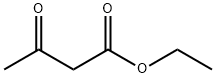
38568-41-1
10 suppliers
inquiry

943749-63-1
27 suppliers
inquiry
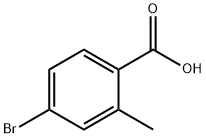
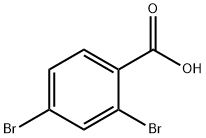
68837-59-2
335 suppliers
$5.00/1g
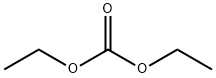
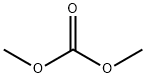
105-58-8
447 suppliers
$14.00/25g

943749-63-1
27 suppliers
inquiry