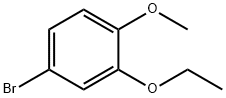
4-BROMO-2-ETHOXY-1-METHOXYBENZENE synthesis
- Product Name:4-BROMO-2-ETHOXY-1-METHOXYBENZENE
- CAS Number:52849-52-2
- Molecular formula:C9H11BrO2
- Molecular Weight:231.09

37942-01-1
244 suppliers
$6.00/5g

75-03-6
369 suppliers
$11.00/5g

52849-52-2
43 suppliers
$45.00/100mg
Yield:52849-52-2 100%
Reaction Conditions:
with potassium carbonate in tetrahydrofuran at 20; for 10.5 h;
Steps:
Application of Synthesis
The first part of the application of synthesis was to synthesize the nucleophilic boronic acids, as shown in the reaction scheme below: (0110) (0111) The hydroxyl group of the 2-methoxyphenol was first acetylated to lower the ability of electrophilic aromatic substitution on 4-position, avoiding the formation of by-products. N-bromosuccinimide (NBS) was then used to selectively conjugate the bromine atom on the 3-position through bromination, followed by hydrolysis of the acetyl group under a basic condition and reacting with diiodoethane to furnish the ethoxy compound 116. The compound 116 was reacted with n-butyl lithium (n-BuLi) and trimethyl borate to form a boronic ester. At last, the target product of boronic acid 117 was obtained through hydrolysis with hydrogen chloride. (0112) The second part was to synthesize the sulfonyl alkyne molecular fragment, as shown in the reaction scheme below. The n-BuLi and trimethylsilylacetylene were used for deprotonation and then reacted with dimethyl sulfide through substitution reaction to furnish the compound 120, followed by treating with meta-chloroperoxybenzoic acid (m-CPBA) to oxidize the sulfide compound to furnish the compound 121. The protecting trimethylsilyl group was removed from the compound 121 through the acid property of silica gel to produce the compound 122.
References:
US11008288,2021,B1 Location in patent:Page/Page column 20-21

64-67-5
382 suppliers
$14.00/5g

37942-01-1
244 suppliers
$6.00/5g

52849-52-2
43 suppliers
$45.00/100mg
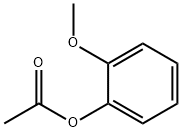
613-70-7
118 suppliers
$30.00/1g

52849-52-2
43 suppliers
$45.00/100mg
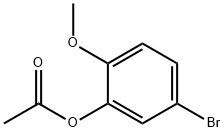
66037-04-5
18 suppliers
$60.00/100mg

52849-52-2
43 suppliers
$45.00/100mg
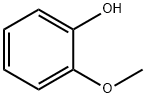
90-05-1
786 suppliers
$5.00/1g

52849-52-2
43 suppliers
$45.00/100mg