
5-Bromo-2-methoxyphenol synthesis
- Product Name:5-Bromo-2-methoxyphenol
- CAS Number:37942-01-1
- Molecular formula:C7H7BrO2
- Molecular Weight:203.03
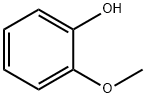
90-05-1
735 suppliers
$5.00/1g

37942-01-1
244 suppliers
$6.00/5g
Yield: 90%
Reaction Conditions:
with sodium hydroxide;N-Bromosuccinimide;potassium tert-butylate;trifluoroacetic anhydride in dichloromethane;acetonitrile
Steps:
1 Preparation of 5-Bromo-2-methoxyphenol
Example 1 Preparation of 5-Bromo-2-methoxyphenol To a solution of guaiacol (5.0 g, 0.04 mol) in acetonitrile (50 ml) at ambient temperature, was added trifluoroacetic anhydride (6.2 ml, 1.1 eq.). The solution was stirred for 5 min., then 1 M potassium tert-butoxide (4.0 ml, 0.1 eq.) was added slowly. The resultant mixture was stirred for 45 min. A solution of N-bromosuccinimide (7.83 g, 1.1 eq.) in acetonitrile (50 ml) was added via addition funnel slowly. The orange solution was stirred for 24 hours then the solvent was removed in rotary evaporator to give a residue which was suspended in dichloromethane (50 ml). A 6 N aqueous solution of sodium hydroxide (20 ml) was added and the organic layer was separated and discarded. The aqueous basic layer was acidified with concentrated hydrochloric acid until pH 2 was reached. Dichloromethane (50 ml) was added to extract the acidic aqueous layer. After being separated, the organic layer was washed with brine and concentrated on rotary evaporator to afford the desired product (8 g) as a redish oil in 90% yield. Data: 1H-NMR (CDCl3) δ ppm: 7.1 (s, 1H, aromatic); 6.95 (d, 1H, aromatic); 6.7 (d, 1H, aromatic); 5.15 (s, 1H, OH); 3.9 (s, 3H, OCH3).
References:
SmithKline Beecham Corporation US6624323, 2003, B1
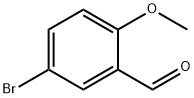
25016-01-7
230 suppliers
$6.00/5g

37942-01-1
244 suppliers
$6.00/5g

98910-57-7
5 suppliers
inquiry

37942-01-1
244 suppliers
$6.00/5g

21702-84-1
153 suppliers
$8.00/5g

37942-01-1
244 suppliers
$6.00/5g
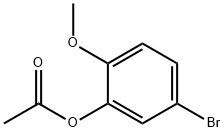
66037-04-5
18 suppliers
$60.00/100mg

37942-01-1
244 suppliers
$6.00/5g