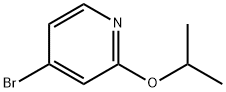
4-broMo-2-isopropoxypyridine synthesis
- Product Name:4-broMo-2-isopropoxypyridine
- CAS Number:1142194-24-8
- Molecular formula:C8H10BrNO
- Molecular Weight:216.08
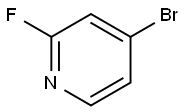
128071-98-7
287 suppliers
$7.00/1g
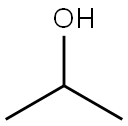
67-63-0
1550 suppliers
$17.00/25ML

1142194-24-8
42 suppliers
$90.00/100mg
Yield:1142194-24-8 83%
Reaction Conditions:
with potassium tert-butylate at 80; for 3 h;Inert atmosphere;
Steps:
15.1
Example 15Synthesis of 3-Methoxy-1-(2-(4-r4-(1-methyl-1 H-? .2.41triazol-3-yl)-phenv?-3,6- dihvdro-?H-pyridin-i-ylV?-oxo-ethvO-pyrrolidine^-carboxylic acid T3-(2- isopropoxy-pyridin-4-yl)-1 H-indazol-5-yll-amide Step 1 :Preparation of 4-Bromo-2-lsopropoxy-pyridine To the stirred solution of 4-bromo-2-fluoro-pyridine 17BV (4.12 g, 23.41 mmole) in 50 mL anhydrous IPA in a 150 mL pressure vessel was added 2.627 g(23.41 mmole) of solid potassium tert-butoxide under dry N2 gas. The pressure vessel was tightly sealed and heated at 80 0C for 3 hours. The pressure vessel was cooled to 0 0C in ice-bath before opening. The contents of the pressure vessel were transferred to 250 mL RBF and concentrated to a small volume. The resulting mixture was partitioned between EtOAc and H2O. The organic phase was separated, washed with saturated NaCI solution and dried over MgSO4. The solvent was evaporated and resulting clear oil was purified on RediSep 80 g cartridge eluting with 20:1 Hexanes/EtOAc to give clear oil 18BV (4.2 g, 83%).
References:
WO2009/105500,2009,A1 Location in patent:Page/Page column 274
73583-37-6
323 suppliers
$9.00/1g

67-63-0
1550 suppliers
$17.00/25ML

1142194-24-8
42 suppliers
$90.00/100mg