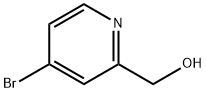
4-Bromo-2-pyridinemethanol synthesis
- Product Name:4-Bromo-2-pyridinemethanol
- CAS Number:131747-45-0
- Molecular formula:C6H6BrNO
- Molecular Weight:188.02
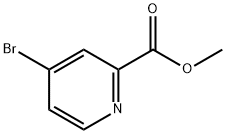
29681-42-3
182 suppliers
$5.00/250mg

131747-45-0
188 suppliers
$13.00/50mg
Yield:131747-45-0 94%
Reaction Conditions:
with sodium tetrahydroborate;ethanol at 20; for 18 h;Inert atmosphere;
Steps:
17
Example 17, Compound 17; (E)-(1 S,14R,15R,18S,21 S)-21 -(3-Hydroxy-benzyl)-18-isopropyl-14-methoxy-15-methyl-3-oxa-6,17,20,23,27-pentaaza-tricyclo[21.3.1.1 *5,9*]octacosa- 5 7,9(28),10-tetraene-2,16,19,22-tetraone; Com ound 17a: (4-Bromo-pyridin-2-yl)-methanol.; Sodium borohydride (763 mg, 20.17 mmol) was added portionwise to a solution of 4- bromo-pyridine-2-carboxylic acid methyl ester (1 .98 g, 9.166 mmol) in ethanol (50 mL) under nitrogen and the reaction mixture was stirred at room temperature for 18 hours. The reaction was quenched by the addition of acetone (10 mL) and the reaction was stirred for 15 minutes. The solvent was evaporated and the residue partitioned between water and ethyl acetate. The aqueous layer was extracted with ethyl acetate and the combined organics were collected, dried over anhydrous sodium sulfate and the solvent evaporated to afford the title compound (1 .61 g, 94%) as a yellow oil. H NMR (300 MHz, CDCI3) 3.4-3.5 {br s, 1 H), 4.80 (s, 2H), 7.40 (dd, J = 5.4, 1 .8 Hz, 1 H), 7.50 (br m, 1 H), 8.39 (d, J = 5.4 Hz, 1 H). LCMS (m/z) 188/200 [M+H], Tr = 1 .55 min.
References:
GILEAD SCIENCES, INC.;SELCIA LIMITED;APPLEBY, Todd;FLIRI, Hans, G.;KEATS, Andrew, J.;LAZARIDES, Linos;MACKMAN, Richard, L.;PETTIT, Simon, N.;POULLENNEC, Karine, G.;SANVOISIN, Jonathan;STEADMAN, Victoria, A.;WATT, Gregory, M. WO2012/78915, 2012, A1 Location in patent:Page/Page column 95-96
100367-74-6
29 suppliers
inquiry

131747-45-0
188 suppliers
$13.00/50mg
30766-03-1
267 suppliers
$7.00/1g

131747-45-0
188 suppliers
$13.00/50mg
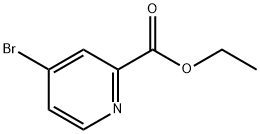
62150-47-4
103 suppliers
$32.00/250mg

131747-45-0
188 suppliers
$13.00/50mg

100367-74-6
29 suppliers
inquiry
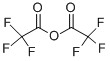
407-25-0
454 suppliers
$9.00/1g

131747-45-0
188 suppliers
$13.00/50mg