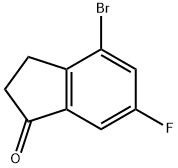
4-BROMO-6-FLUOROINDAN-1-ONE synthesis
- Product Name:4-BROMO-6-FLUOROINDAN-1-ONE
- CAS Number:174603-56-6
- Molecular formula:C9H6BrFO
- Molecular Weight:229.05

174603-55-5
59 suppliers
$51.00/100mg

174603-56-6
70 suppliers
$9.00/100mg
Yield: 71%
Reaction Conditions:
Stage #1:3-(2-bromo-4-fluoro-phenyl)-propionic acid with oxalyl dichloride in dichloromethane at 20; for 16 h;Inert atmosphere;
Stage #2: with aluminum (III) chloride in dichloromethane at 20; for 3 h;
Steps:
Step 4
Oxalyl Chloride (33 mL, 381.78 mmol, 2 eq) was added to a stirred solution of 3-(2-bromo-4-fluoro-phenyl)- propionic acid (47.15 g, 190.89 mmol, 1 eq) in dry DCM (500 mL) under inert atmosphere, and the reaction mixture was stirred at RT for 16 h. After completion (monitored by TLC), DCM and oxalyl chloride were removed by rotary evaporator under inert atmosphere and the crude material was carried on to the next step without purification. The cmde material was dissolved in dry DCM (500 mL), a suspension of anhydrous AICl3( 38.4 g, 286.33 mmol, 1.5 eq) in DCM (200 mL) was added at RT, and the reaction mixture was refluxed for 3 h. After completion, the reaction mixture was poured into ice-cold water (1000 mL) and the organic fraction was separated. The aqueous layer was extracted with DCM (2 x 500 mL), and the combined organic layers were washed with water (500 mL) and brine (500mL) , dried over anhydrous Na2SO4 and concentrated under reduced pressure to afford 4-bromo-6-fluoro-indan- 1-one (31 g,71%).
References:
GRÜNENTHAL GMBH WO2021/144439, 2021, A1 Location in patent:Page/Page column 86; 87
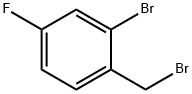
61150-57-0
137 suppliers
$8.00/1g

174603-56-6
70 suppliers
$9.00/100mg
![Propanedioic acid, 2-[(2-bromo-4-fluorophenyl)methyl]-, 1,3-diethyl ester](/CAS/20210305/GIF/174603-54-4.gif)
174603-54-4
0 suppliers
inquiry

174603-56-6
70 suppliers
$9.00/100mg
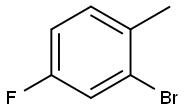
1422-53-3
331 suppliers
$5.00/5g

174603-56-6
70 suppliers
$9.00/100mg