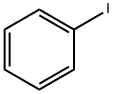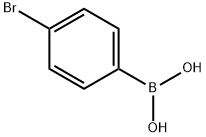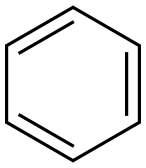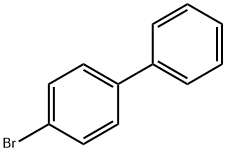
4-Bromobiphenyl synthesis
- Product Name:4-Bromobiphenyl
- CAS Number:92-66-0
- Molecular formula:C12H9Br
- Molecular Weight:233.1
(i) 15.4 g biphenyl (0.1 M);
(ii) 100 ml solvent
at ambient temperature Then, over 10 min, the bromine was poured therein (39.2 g/0.22 M), whereupon the mixture was agitated for a varying period of time at ambient temperature. Upon completion of the reaction, the excess bromine was consumed by sodium or potassium sulfite or bisulfite. The organic phase was washed, dried and diluted to a given volume, and analyzed by CPG to give 4-Bromobiphenyl.
5122-94-1
390 suppliers
$5.00/1g

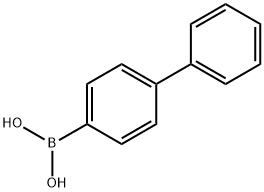
92-66-0
551 suppliers
$10.00/1g
Yield:92-66-0 99%
Reaction Conditions:
with sodium nitrite in acetonitrile at 80; for 1 h;Sealed tube;
Steps:
General procedure for the ipso-bromination of arylboronic acids
General procedure: In a pressure tube, to a solution of a selected arylboronic acid (0.25 mmol) and sodium nitrate (4.3 mg, 0.063 mmol) in dry acetonitrile (2 mL), PVP-Br 2 (1:1) (89 mg, 0.34 mmol) was added. The reaction mixture was stirred vigorously at 80 C for required time. Upon completion, the reaction mixture was diluted with dichloromethane and filtered through celite, washing with more dichloromethane. The filtrate was washed with Na2S2O4 solution, saturated NaCl solution and dried over anhydrous Na2SO4. Removal of the solvent under reduced pressure afforded the product in pure form, as verified by 1H NMR and 13C NMR.
References:
Fu, Fang;Gurung, Laxman;Czaun, Miklos;Mathew, Thomas;Prakash, G.K. Surya [Tetrahedron Letters,2019,vol. 60,# 38,art. no. 151020] Location in patent:supporting information

92-52-4
384 suppliers
$9.00/5g

92-66-0
551 suppliers
$10.00/1g