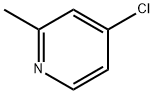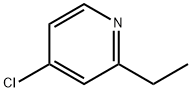
4-chloro-2-ethylpyridine synthesis
- Product Name:4-chloro-2-ethylpyridine
- CAS Number:3678-65-7
- Molecular formula:C7H8ClN
- Molecular Weight:141.6
Yield:3678-65-7 24%
Reaction Conditions:
Stage #1: 4-chIoro-2-methylpyridinewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexanes at -70 - -20;
Stage #2: methyl iodide in tetrahydrofuran;hexanes at -70 - 20;
Steps:
12
To a stirred solution of diisopropylamine (0.86 ml, 6.1 mmol) in THF (15 ml) at -2O0C was added dropwise a solution of n-butyllithium in hexanes (1.2M, 5.07 ml, 6.1 mmol). The reaction mixture was stirred at -2O0C for 20 minutes and was then cooled to -7O0C. A solution of 2-methyl-4-chloropyridine (0.74 g, 5.8 mmol) in THF (10 ml) was added dropwise over 20 minutes and the reaction mixture was stirred for 1 hour at -7O0C. Methyl iodide (0.38 ml, 6.1 mmol) was then added dropwise and the reaction was allowed to warm to room temperature with stirring. The solvent was evaporated at reduced pressure and the resulting residue purified by FCC. 1H NMR indicated a -2.2 : 1 mixture of 2-ethyl and 2-(l-methylethyl) compounds. Re-purification by silica FCC [pentane / ether eluting with gradient 9:1 to 1 :1] gave the title compound (209 mg, 24%) as colourless oil. LCMS data: The product did not ionise, 99% UV at Rt = 4.34 mins observed (High pH method).NMR data: 1H NMR (250 MHz, CDCl3) δ ppm 8.43 (1 H, d, J=5.3 Hz), 7.19 (1 H, d, J=2.0 Hz), 7.13 (1 H, dd, J=5.3, 2.0 Hz), 2.82 (2 H, q, J=7.6 Hz), 1.31 (3 H, t, J=7.6 Hz).
References:
WO2009/135842,2009,A1 Location in patent:Page/Page column 73-74
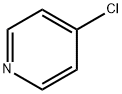
626-61-9
48 suppliers
$365.00/500mg

3678-65-7
9 suppliers
inquiry
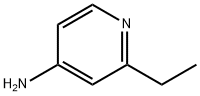
50826-64-7
43 suppliers
$45.00/50mg

3678-65-7
9 suppliers
inquiry