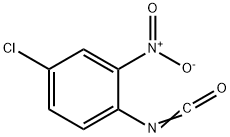
4-CHLORO-2-NITROPHENYL ISOCYANATE synthesis
- Product Name:4-CHLORO-2-NITROPHENYL ISOCYANATE
- CAS Number:28162-63-2
- Molecular formula:C7H3ClN2O3
- Molecular Weight:198.56
Yield:-
Reaction Conditions:
in ethyl acetate at 0; for 18 h;Heating / reflux;
Steps:
A6
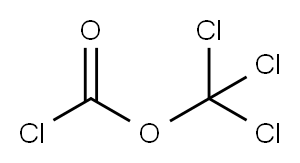
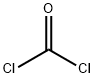
Compound A6 WAS prepared via the isocyanate from commercially available 4-chloro-2- nitro-phenylamine [CAS-No. 89-63-4] (5.0 g, 29 mmol) with diphosgene (1.75 mL, 14.5 mmol) in EtOAc (60 mL), followed by treatment with TERT-BUOH (30 mL) in CH2CL2 (60 mL) according to the general procedure A (method a). Method a (from 2-nitroanilines): To a solution of diphosgene (4.1 mL, 34.1 mmol) in EtOAc (40 mL) at 0°C WAS added a solution of the 2-nitroaniline (45.5 mmol) in EtOAc (200-500 mL), and the mixture was heated to reflux for 18 h. The solvent was removed in vacuum to leave a brown solid, which was triturated with hot hexane (200 mL). The solid material was filtered off and the filtrate was concentrated under reduced pressure to leave the pure 2-nitrophenylisocyanate as a yellow solid. This material was refluxed in a mixture of excess tert-BuOH in CH2CL2 FOR 2.5 h. Removal of the solvent left an orange solid which was purified by silica gel column chromatography with hexane/EtOAc to give the (2-nitro-phenyl) -carbamic acid tert-butyl ester as a yellow solid.
References:
WO2005/14002,2005,A1 Location in patent:Page/Page column 28; 34

7153-86-8
20 suppliers
inquiry

28162-63-2
22 suppliers
$172.00/1g
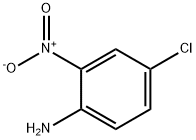
89-63-4
1 suppliers
$13.00/50g

28162-63-2
22 suppliers
$172.00/1g