
4-Chloro-3-Methyl-3H-iMidazo[4,5-c]pyridine synthesis
- Product Name:4-Chloro-3-Methyl-3H-iMidazo[4,5-c]pyridine
- CAS Number:87034-78-4
- Molecular formula:C7H6ClN3
- Molecular Weight:167.6
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
134 suppliers
$19.00/250mg

74-88-4
349 suppliers
$15.00/10g
![4-CHLORO-1-METHYL-1H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/50432-68-3.gif)
50432-68-3
86 suppliers
$38.00/250mg
![4-Chloro-3-Methyl-3H-iMidazo[4,5-c]pyridine](/CAS/20150408/GIF/87034-78-4.gif)
87034-78-4
31 suppliers
$389.00/1g
Yield: 24% , 19%
Reaction Conditions:
Stage #1:4-chloro-3H-imidazo[4,5-c]pyridine with sodium hexamethyldisilazane in tetrahydrofuran;dimethyl sulfoxide at 20; for 1 h;Inert atmosphere;
Stage #2:methyl iodide in tetrahydrofuran;dimethyl sulfoxide at 20; for 11 h;
Steps:
a 4-chloro-l-methyl- imidazo[4,5-clpyridine, Intermediate DG and 4-chloro-3-methyl-3j/-imidazo[4.,5-c|pyridine, Intermediate DH Step a:
To a solution of 4-chloro-3i7-imidazo[4,5-c]pyridine (1 g, 6.51 mmol, CAS 81053- 66-9) in DMSO/THF (2.5 mL/25 mL) was added NaHMDS (9.76 mL, 9.76 mmol) at 20 °C under N2. The reaction mixture was stirred at 20 °C for 1 h. Then, to the reaction mixture was added Mel (1.21 mL, 19.5 mmol) at 20 °C. The reaction mixture was then stirred at 20 °C for 11 h. The reaction mixture was filtered and the filtrate was concentrated to give a residue. The residue was purified by prep-HPLC (NH3 H20) to give 4-chloro-l -methyl- H- imidazo[4,5-c]pyridine (Intermediate DG) (257.2 mg, 24% yield) as a white solid, LCMS (ESI+) m/z: 167.8 (M+H)+; 1HNMR (400MHz, DMSO-i) d 8.16-8.14 (m, 1H), 7.92 (s, 1H), 7.27-7.25 (m, 1H), 3.71 (s, 3H), and 4-chloro-3-methyl-3if-imidazo[4,5-c]pyridine (Intermediate DH) (201.8 mg, 19% yield) as a white solid, LC-MS (ESI+) m/z: 167.8 (M+H)+, 1HNMR (400MHz, DMSO-i) d 8.34-8.13 (m, 1H), 7.98 (m, 1H), 7.79-7.54 (m, 1H), 7.29 (m, 1H), 4.34-4.09 (m, 3H).
References:
RELAY THERAPEUTICS, INC.;D.E. SHAW RESEARCH, LLC;TAYLOR, Alexander, M.;LESCARBEAU, André;KELLEY, Elizabeth, H.;SHORTSLEEVES, Kelley, C.;WALTERS, W., Patrick;MURCKO, Mark, Andrew;MCLEAN, Thomas, H.;GUNAYDIN, Hakan;GIORDANETTO, Fabrizio;THERRIEN, Eric WO2019/183367, 2019, A1 Location in patent:Paragraph 0276
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
134 suppliers
$19.00/250mg

74-88-4
349 suppliers
$15.00/10g
![4-Chloro-3-Methyl-3H-iMidazo[4,5-c]pyridine](/CAS/20150408/GIF/87034-78-4.gif)
87034-78-4
31 suppliers
$389.00/1g
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
134 suppliers
$19.00/250mg
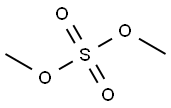
77-78-1
305 suppliers
$19.69/1ml
![4-CHLORO-1-METHYL-1H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/50432-68-3.gif)
50432-68-3
86 suppliers
$38.00/250mg
![4-Chloro-3-Methyl-3H-iMidazo[4,5-c]pyridine](/CAS/20150408/GIF/87034-78-4.gif)
87034-78-4
31 suppliers
$389.00/1g
![4-CHLORO-1-H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/2770-01-6.gif)
2770-01-6
134 suppliers
$19.00/250mg

74-88-4
349 suppliers
$15.00/10g
![4-CHLORO-1-METHYL-1H-IMIDAZO[4,5-C]PYRIDINE](/CAS/GIF/50432-68-3.gif)
50432-68-3
86 suppliers
$38.00/250mg
![4-Chloro-3-Methyl-3H-iMidazo[4,5-c]pyridine](/CAS/20150408/GIF/87034-78-4.gif)
87034-78-4
31 suppliers
$389.00/1g