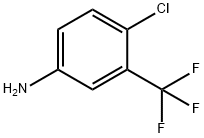
(4-Chloro-3-trifluoromethyl-phenyl)-methyl-amine synthesis
- Product Name:(4-Chloro-3-trifluoromethyl-phenyl)-methyl-amine
- CAS Number:42265-69-0
- Molecular formula:C8H7ClF3N
- Molecular Weight:209.6
Yield:42265-69-0 64%
Reaction Conditions:
Stage #1: 4-chloro-3-trifluoromethyl-anilinewith pyridine;copper (II) acetate in 1,4-dioxane at 20; for 0.25 h;
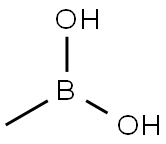
Stage #2: dihydroxy-methyl-borane in 1,4-dioxane at 110; for 4 h;
Steps:
3 1-(4-Chloro-3-(trifluoromethyl)phenyl)-3-(6-chlorobenzo[d]thiazol-2-yl)-1-methylurea (I-3)
Copper(II) acetate (3.49 g, 19.23 mmol) was added to 2-chloro-5-aminotrifluorotoluene II-3 (1.50 g, 7.69 mmol) at room temperatureThe solution of pyridine (2.17 mL, 2.13 g, 26.92 mmol) and 1,4-dioxane (70 mL) was stirred at the same temperature for 15 min.Methylboronic acid (1.15 g, 19.23 mmol) was added, and the reaction was carried out under reflux at an internal temperature of 110 °C for 4 h. Turn off heat and cool to room temperature.After filtration, the filtrate was concentrated under reduced pressure at 60°C to obtain a black crude product. Silica gel column separation and purification [eluent:Petroleum ether: ethyl acetate volume ratio=60:125:1 gradient elution] to obtain 4-chloro-N-methyl-3-(trifluoromethyl)aniline III-3 (1.03g, 64.0%) .
References:
CN114349707,2022,A Location in patent:Paragraph 0138-0144
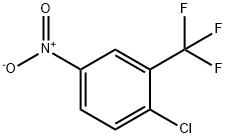
777-37-7
298 suppliers
$10.00/5g

42265-69-0
11 suppliers
inquiry