
4-CHLORO-4'-METHOXYBIPHENYL synthesis
- Product Name:4-CHLORO-4'-METHOXYBIPHENYL
- CAS Number:58970-19-7
- Molecular formula:C13H11ClO
- Molecular Weight:218.68

637-87-6
288 suppliers
$38.00/10g
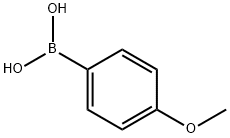
5720-07-0
457 suppliers
$6.00/5g

58970-19-7
34 suppliers
$209.00/1g
Yield:58970-19-7 98%
Reaction Conditions:
with potassium carbonate in ethanol;water at 50; for 1 h;Suzuki-Miyaura Coupling;
Steps:
2.4.1 Suzuki Cross-Coupling Reaction
General procedure: A flame-dried 50mL round-bottom flask equipped with amagnetic stir bar and a rubber septum was charged with arylhalide (1.0mmol), phenyl boronic acid (1.1mmol), K2CO3(2.0mmol) and CL-salen-Pd(II) (0.5% mmol). The mixturewas stirred in Ethanol: H2O= 1:1 (5.0mL) at 50 underair atmosphere for 1h. The mixture was cooled to roomtemperature, quenched with water (5mL), and diluted withethyl acetate (5mL). The layers were separated, and theaqueous layer was extracted with 2 × 5mL of ethyl acetate.The combined organic extracts were dried over anhydrousmagnesium sulfate, filtered, and concentrated in vacuo.Finally, the product was purified by column chromatography.
References:
Sun, Peng;Yang, Jiaojiao;Chen, Chunxia;Xie, Kaijun;Peng, Jinsong [Catalysis Letters,2020,vol. 150,# 10,p. 2900 - 2910]
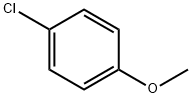
623-12-1
273 suppliers
$10.00/25g
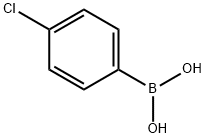
1679-18-1
589 suppliers
$10.00/1g

58970-19-7
34 suppliers
$209.00/1g
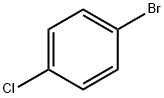
106-39-8
444 suppliers
$14.00/5g

5720-07-0
457 suppliers
$6.00/5g

58970-19-7
34 suppliers
$209.00/1g
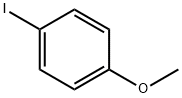
696-62-8
349 suppliers
$5.00/1g

1679-18-1
589 suppliers
$10.00/1g

58970-19-7
34 suppliers
$209.00/1g
