
4-CHLORO-5,7-DIMETHOXYQUINAZOLINE synthesis
- Product Name:4-CHLORO-5,7-DIMETHOXYQUINAZOLINE
- CAS Number:884340-91-4
- Molecular formula:C10H9ClN2O2
- Molecular Weight:224.64
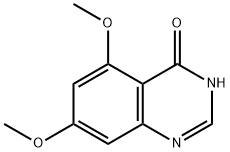
379228-27-0
34 suppliers
$45.00/10mg

884340-91-4
36 suppliers
$65.00/5mg
Yield:884340-91-4 52%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;trichlorophosphate in acetonitrile; for 2 h;Heating / reflux;
Steps:
1.a
A solution of 5,7-dimethoxyquinazolin-4(3H)-one (5.0g, 24mmol) (for synthesis see: Ηennequin, Laurent Francois Andre; PIe, Patrick. Preparation of 4-anilinoquinazoline derivatives for the treatment of tumors. PCT Int. Appl. (2001), WO 01/094341), di-iso- propylethylamine (13.5ml, 77.7mmol) and phosphorus oxychloride (7.24ml, 77.7mmol) in acetonitrile (125ml) was heated under reflux for 2 hours. The mixture was evaporated in vacuo and the residue was partitioned between dichloromethane and a saturated solution of sodium hydrogencarbonate. The organic layer was separated and washed with a solution of brine, dried over magnesium sulphate and then evaporated to leave a dark brown solid. The crude product was purified by flash chromatography on silica eluting with ethylacetate to give 4-chloro-5,7-dimethoxyquinazoline (2.83g, 52% yield): 1H-NMR (DMSO d6): 8.81 (s, IH), 7.01 (d, IH), 6.83 (d, IH), 3.96 (s, 3H), 3.95 (s, 3H). EPO
References:
WO2006/67391,2006,A1 Location in patent:Page/Page column 49-50

21577-57-1
112 suppliers
$20.00/250mg

884340-91-4
36 suppliers
$65.00/5mg
379228-26-9
27 suppliers
inquiry

884340-91-4
36 suppliers
$65.00/5mg