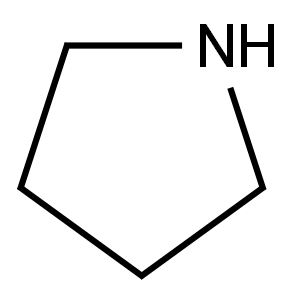
4-CHLORO-5-NITRO-6-(1-PYRROLIDINYL)PYRIMIDINE synthesis
- Product Name:4-CHLORO-5-NITRO-6-(1-PYRROLIDINYL)PYRIMIDINE
- CAS Number:25710-26-3
- Molecular formula:C8H9ClN4O2
- Molecular Weight:228.64
Yield:25710-26-3 67.3%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);potassium carbonate;(R)-2,2'-bis(diphenylphosphanyl)-1,1'-binaphthyl in toluene at 25; for 3.5 h;Inert atmosphere;
Steps:
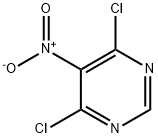
4.1 General procedure for reaction of amines with 4,6-dichloro-5-nitropyrimidine (3a-3o)
General procedure: To a stirred solution of 4,6-dichloro-5-nitropyrimidine (1.5mmol), amine (0.5mmol), Pd2(dba)3 (0.01mmol), R-BINAP (0.03mmol) and potassium carbonate (0.7mmol) in toluene (5mL) at room temperature and the mixture was under an argon atmosphere for 3.5h. The resulting reaction mixture was filtered and evaporated. The residue was purified by column chromatography on silica gel.
References:
Liu, Meng-Meng;Mei, Qiong;Zhang, Yi-Xiao;Bai, Peng;Guo, Xiang-Hai [Chinese Chemical Letters,2017,vol. 28,# 3,p. 583 - 587]