
4-chloro-6-(1-methylethyl)pyrimidine synthesis
- Product Name:4-chloro-6-(1-methylethyl)pyrimidine
- CAS Number:954222-10-7
- Molecular formula:C7H9ClN2
- Molecular Weight:156.61
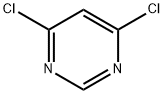
1193-21-1
555 suppliers
$16.00/5g
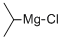
1068-55-9
319 suppliers
$20.00/25ml

954222-10-7
27 suppliers
$149.00/100mg
Yield:954222-10-7 25%
Reaction Conditions:
with iron(III)-acetylacetonate in tetrahydrofuran;1-methyl-pyrrolidin-2-one at 0; for 2 h;
Steps:
48.1 Step 1: Example 48b
To a mixture of Example 48a (1.5 g, 10 mmol) in NMP ( 5 mL ) were added Tetrahydrofuran (1185) (50 mL), and Fe(acac)3 (353 mg, 1.0 mmol). The solution was cooled to 0°C and i-PrMgCl (10 mL, 2N) was added slowly at 0°C. The solution was stirred at 0°C for 2 h, which was then extracted with EtOAc (50 mL*3), washed with brine, dried over Na2S04, and filtered. The filtrate was concentrated, and the residue was purified by column chromatography (silica gel, Petroleum Ether/EtOAc = 1/0 ~ 20/1) to give Example 48b (400 mg, yield 25%) as yellow oil. LCMS [M+l]+ = 156.9
References:
WO2019/240938,2019,A1 Location in patent:Paragraph 00447