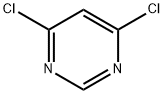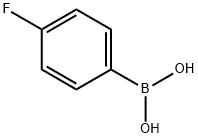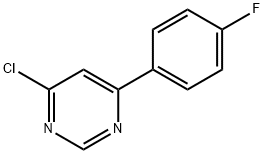
4-Chloro-6-(4-fluoro-phenyl)-pyrimidine synthesis
- Product Name:4-Chloro-6-(4-fluoro-phenyl)-pyrimidine
- CAS Number:85979-61-9
- Molecular formula:C10H6ClFN2
- Molecular Weight:208.62
Yield:85979-61-9 41.1%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium carbonate in 1,4-dioxane;water at 120; for 8 h;Sealed tube;Inert atmosphere;
Steps:
2.A A, 4-chloro-6- (4 '-fluorophenyl) pyrimidine;
2 g (0.014 mol) of 4-fluorobenzeneboronic acid was weighed,4,6-dichloropyrimidine (2.14 g, 0.0142 mol)Pd (dppf) CI20.15g and anhydrous potassium carbonate 2.5 g were placed in a 120 mL sealed tube,A mixed solution of 60 mL of dioxane and 20 mL of deionized water was placed as a solvent in a sealed tube,Add the stirrup and cover the stopper.The whole device to take a vacuum nitrogen replacement 3 ~ 4 times,Placed in an oil bath and heated to 120 ° C for about 8 hours with stirring on a magnetic stirrer.After the reaction, the tube is cooled toThe solution in the sealed tube was placed in a rotary vial, and the tetrahydrofuran solvent was distilled off under reduced pressure by rotary evaporator. And then BAcid ethyl ester and water extraction of organic products 3 ~ 4 times, vacuum distillation of solvent to petroleum ether: dichloromethane 1: 1 will be steamed after the productThe column was separated by column chromatography and separated by separate column chromatography using dichloromethane to give 1.22 g of a white powder,41.1%.
References:
CN106632488,2017,A Location in patent:Paragraph 0109-0111