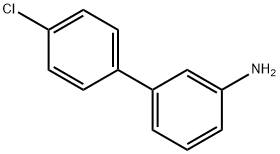
4'-CHLORO-BIPHENYL-3-YLAMINE synthesis
- Product Name:4'-CHLORO-BIPHENYL-3-YLAMINE
- CAS Number:56970-11-7
- Molecular formula:C12H10ClN
- Molecular Weight:203.67
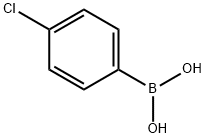
1679-18-1
610 suppliers
$10.00/1g
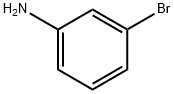
591-19-5
332 suppliers
$10.00/10g

56970-11-7
38 suppliers
$120.00/1g
Yield:56970-11-7 70%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in methanol at 100; for 2.5 h;Inert atmosphere;Microwave irradiation;Suzuki coupling;
Steps:
1.1. General Procedure for Suzuki coupling reactions
General procedure: To a solution of phenylboronic acid (1 equiv.) in MeOH were added, Na2CO3 (2 equiv.) and 3-bromoaniline (1 equiv.). Pd(OAc)2 or Pd(PPh3)4 (equiv. varied) was added and the reaction mixture heated at reflux for several hours at 70 °C or stirring in a microwave at 100 °C. The solution was then cooled to r.t, diluted with methanol and filtered through a pad of celite to remove the black precipitate. The filtrate was then concentrated in vacuo and the residual product was then partitioned between water (15 mL) and DCM (15 mL). The organic phase was removed and the aqueous phase was further extracted with DCM (2 × 15 mL). The organic fractions were combined, dried over anhydrous MgSO4, filtered and evaporated to dryness to give the crude product. The resulting oily residue was purified using flash column chromatography and the major product fractions were evaporated to dryness to afford the title compound. The following compounds were prepared in this manner.
References:
Szabo, Monika;Agostino, Mark;Malone, Daniel T.;Yuriev, Elizabeth;Capuano, Ben [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 22,p. 6782 - 6787] Location in patent:supporting information; experimental part

1679-18-1
610 suppliers
$10.00/1g
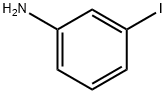
626-01-7
262 suppliers
$8.00/5g

56970-11-7
38 suppliers
$120.00/1g
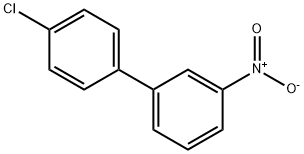
54044-92-7
1 suppliers
inquiry

56970-11-7
38 suppliers
$120.00/1g

13331-27-6
353 suppliers
$5.00/1g

56970-11-7
38 suppliers
$120.00/1g

637-87-6
297 suppliers
$38.00/10g

56970-11-7
38 suppliers
$120.00/1g