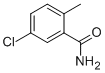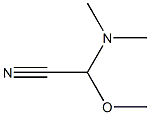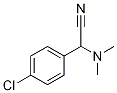
4-Chloro-α-(dimethylamino)benzeneacetonitrile synthesis
- Product Name:4-Chloro-α-(dimethylamino)benzeneacetonitrile
- CAS Number:15190-08-6
- Molecular formula:C10H11ClN2
- Molecular Weight:194.66
Yield:15190-08-6 94%
Reaction Conditions:
with anhydrous sodium carbonate in tetrahydrofuran;lithium hydroxide monohydrate at 25; for 1 h;Inert atmosphere;
Steps:
5.1.4 General procedure of amination
General procedure: Na2CO3 (1.00 equiv.) and dimethylamine (1.50 equiv., 40% in aqueous) were added to a stirred solution of 2-bromo-2-phenylacetonitriles (1.00 equiv.) in THF at 25°C. The reaction mixture was stirred at 25°C for 1h, and monitored by TLC. After reaction completion, the reaction mixture was quenched with water and diluted with ethyl acetate, washed with water, brine. The organic layer was dried over anhydrous MgSO4(s), and concentrated under reduced pressure. The crude product was purified by Isco Combi-Flash Companion column chromatography to give the desired product.
References:
Kuppusamy, Ramajayam;Hsu, Ying-Ting;Ke, Yi-Yu;Chang, Po-Wei;Chang, Yung-Chiao;Chang, Hsiao-Fu;Wang, Pei-Chen;Lin, Yu-Hao;Huang, Yu-Chen;Yeh, Teng-Kuang;Chuang, Jian-Ying;Loh, Horace H.;Shih, Chuan;Chen, Chiung-Tong;Yeh, Shiu-Hwa;Ueng, Shau-Hua [European Journal of Medicinal Chemistry,2022,vol. 243,art. no. 114728]
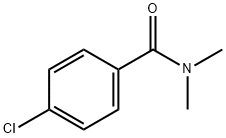
14062-80-7
47 suppliers
$15.00/5mg

15190-08-6
18 suppliers
inquiry
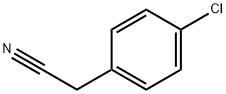
140-53-4
476 suppliers
$13.00/25g

15190-08-6
18 suppliers
inquiry
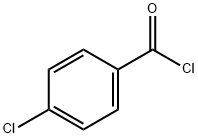
122-01-0
370 suppliers
$13.00/25g

15190-08-6
18 suppliers
inquiry