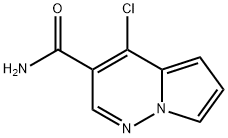
4-chloropyrrolo[1,2-b]pyridazine-3-carboxamide synthesis
- Product Name:4-chloropyrrolo[1,2-b]pyridazine-3-carboxamide
- CAS Number:1400688-75-6
- Molecular formula:C8H6ClN3O
- Molecular Weight:195.61
![4-chloropyrrolo[1,2-b]pyridazine-3-carboxylic acid](/CAS/20180629/GIF/1400688-73-4.gif)
1400688-73-4
12 suppliers
inquiry
![4-chloropyrrolo[1,2-b]pyridazine-3-carboxamide](/CAS/20180703/GIF/1400688-75-6.gif)
1400688-75-6
6 suppliers
inquiry
Yield:1400688-75-6 93%
Reaction Conditions:
Stage #1: 4-chloropyrrolo[1,2-b]pyridazine-3-carboxylic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 1.5 h;Cooling with ice;
Stage #2: with ammonia in dichloromethane at 0; for 1.5 h;
Steps:
3
Alternative preparation of 4-chloropyrrolo[l, 2-b] pyridazine-3-carboxamide (Preparation 3); [00165] Slurried 4-chloropyrrolo[l,2-b]pyridazine-3-carboxylic acid (Preparation 1 from Step 6, 0.4 g, 2.04 mmol) in dichloromethane (6 mL) and cooled in ice bath and successively added oxalyl chloride (0.534 mL, 6.10 mmol) and DMF (0.01 mL, 0.129 mmol). Allowed slurry to warm to rt and stir for 1.5 h giving a clear, yellow- green solution. The reaction was concentrated under vacuum to yield a yellow solid which was redissolved in dichloromethane (10 mL) and reconcentrated to remove all residual oxalyl chloride. This solid was dissolved in dichloromethane (6 mL) and the solution was added dropwise via pipette into a well-stirred ammonia indichloromethane solution (prepared by extracting 15 mL of cone aq. ammonium hydroxide with 3 x 10 mL portions of dichloromethane) at 0 °C. After stirring for 1.5 h at 0 °C, water was added to dissolve most solids (~ 15 mL) and the layers were separated and the aqueous portion was extracted with additional dichloromethane. The combined extracts were washed with brine, dried over anhyd. Na2S04, decanted and concentrated on rotovap to yield 370 mg (93%) of a yellow solid as the title compound. Material was used as is without any further purification. LCMS(condition B): m/z 195, 197. HPLC (condition B): retention time = 1.75 min.
References:
WO2012/125887,2012,A1 Location in patent:Page/Page column 67
![Pyrrolo[1,2-b]pyridazine-3-carboxylic acid, 4-hydroxy-, ethyl ester](/CAS/20210111/GIF/1260849-93-1.gif)
1260849-93-1
2 suppliers
inquiry
![4-chloropyrrolo[1,2-b]pyridazine-3-carboxamide](/CAS/20180703/GIF/1400688-75-6.gif)
1400688-75-6
6 suppliers
inquiry
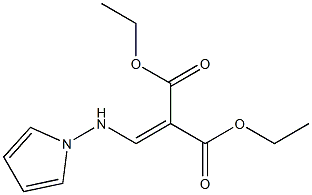
156335-36-3
1 suppliers
inquiry
![4-chloropyrrolo[1,2-b]pyridazine-3-carboxamide](/CAS/20180703/GIF/1400688-75-6.gif)
1400688-75-6
6 suppliers
inquiry
![ethyl 4-chloropyrrolo[1,2-b]pyridazine-3-carboxylate](/CAS/20180703/GIF/1400688-74-5.gif)
1400688-74-5
18 suppliers
inquiry
![4-chloropyrrolo[1,2-b]pyridazine-3-carboxamide](/CAS/20180703/GIF/1400688-75-6.gif)
1400688-75-6
6 suppliers
inquiry
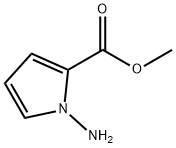
122181-85-5
51 suppliers
inquiry
![4-chloropyrrolo[1,2-b]pyridazine-3-carboxamide](/CAS/20180703/GIF/1400688-75-6.gif)
1400688-75-6
6 suppliers
inquiry