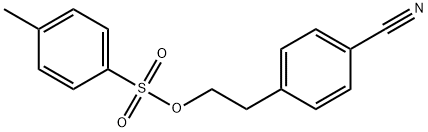
4-Cyanophenethyl 4-Methylbenzenesulfonate synthesis
- Product Name:4-Cyanophenethyl 4-Methylbenzenesulfonate
- CAS Number:80632-27-5
- Molecular formula:C16H15NO3S
- Molecular Weight:301.36
Yield:80632-27-5 66%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
Steps:
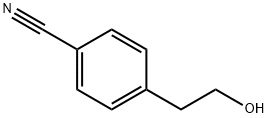
34 GENERAL PROCEDURE 34; Preparation of 4- (2-Aminoethyl) benzonitrile (169).
Benzonitrile 166 (Alfa, 41283) (2g, 13.6 mmol) was dissolved in CH2C12 (30 mL), and Et3N (3.62 mL, 26 mmol) was added. The reaction mixture was chilled to 0 °C and tosyl chloride (3.89 g, 20 mmol) was added as a solid. The reaction was allowed to warm to rt overnight while being stirred under a positive pressure of nitrogen. After stirring for 17h, reaction was quenched by addition of a saturated, aqueous solution of NH4CL, and the mixture was extracted with CH2CL2. The organic solution was separated and rinsed with NAHC03 and brine before drying over NA2S04. The drying agent was filtered off and the filtrate was concentrated under reduced pressure to give a yellow solid as the crude product. This solid was recrystallized from EtOAc/hexanes to give 167 (2.71 g, 66% yield) as a white solid.
References:
WO2004/98589,2004,A1 Location in patent:Page 174