
4-(Dichloromethyl)anisole synthesis
- Product Name:4-(Dichloromethyl)anisole
- CAS Number:21185-25-1
- Molecular formula:C8H8Cl2O
- Molecular Weight:191.05
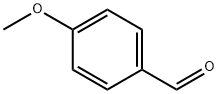
123-11-5
841 suppliers
$10.00/10g

21185-25-1
1 suppliers
inquiry
Yield:21185-25-1 95%
Reaction Conditions:
with polystyrene supported chloro diphenylphsophonium chloride in chloroform at 60; for 18 h;Appel Halogenation;
Steps:
Typical procedure for the deoxychlorination and dehydration reactions
General procedure: To asuspension of PSP Cl or PSP Br (6 equiv) in CHCl3 or DCE (20 mL) was added the appropriate substrate(s) e.g. alcohol/aldehyde/epoxide/oxime/carboxylicacid/amine (1 equiv). The mixture was heated at either 60°C (CHCl3 reactions) or 75°C (DCE reactions) for 18 h. The reaction mixture was filtered and the filter cake washed with CH2Cl2 (2 10 mL). The solvent was removed in vacuo to afford the corresponding products, which were either judged pure by 1H NMR analysis of the crude reaction product or were purified via normal phase silica gel flash column chromatography eluting with a Et2O/petroleum ether 40-60 solvent mixture. The recovered polymer could be reused without anyfurther manipulation.
References:
Tang, Xiaoping;An, Jie;Denton, Ross M. [Tetrahedron Letters,2014,vol. 55,# 4,p. 799 - 802]
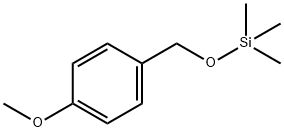
14629-56-2
1 suppliers
inquiry

21185-25-1
1 suppliers
inquiry
![2H-Pyran, tetrahydro-2-[(4-methoxyphenyl)methoxy]-](/CAS/20210305/GIF/18494-82-1.gif)
18494-82-1
1 suppliers
inquiry

21185-25-1
1 suppliers
inquiry
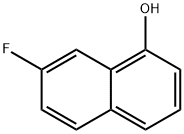
3132-92-1
62 suppliers
$119.00/50mg

21185-25-1
1 suppliers
inquiry

123-11-5
841 suppliers
$10.00/10g

123-11-5
841 suppliers
$10.00/10g
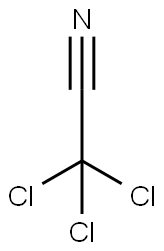
545-06-2
230 suppliers
$10.00/5g

21185-25-1
1 suppliers
inquiry
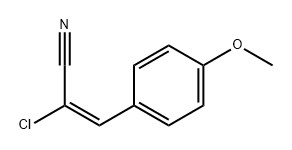
71707-37-4
0 suppliers
inquiry
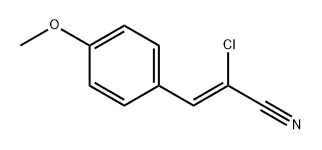
908864-31-3
0 suppliers
inquiry