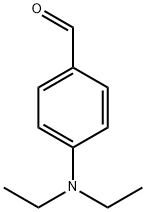
4-Diethylaminobenzaldehyde synthesis
- Product Name:4-Diethylaminobenzaldehyde
- CAS Number:120-21-8
- Molecular formula:C11H15NO
- Molecular Weight:177.24
Yield:120-21-8 92%
Reaction Conditions:
with Aliquat 336;potassium carbonate in dimethyl sulfoxide at 95; for 72 h;
Steps:
(E)-(4-diethylanilinostyryl)pyridine (L)
4-fluorobenzaldehyde (2.5 g, 0.02 mol), diethyl amine(5.12 g, 0.07 mol), K2CO3 (4.15 g), DMSO (25.0 mL) and Aliquat-336 (0.05 mL) were added into a one-necked flask (100 mL) fitted with a stirrer and a condenser. The mixture was stirred for 72 h at 95C, and then cooled to room temperature. The yellow precipitation was obtained after ice water wasintroduced into the flask. The crude product was washed with cool water for 2-3 times, and thenrecrystallized from ethanol. Yellow solid (4-diethylaminobenzaldehyde) was obtained. Yield: 92%.Anal. Calcd. for C11H15NO: C, 74.54; H, 8.53; N, 7.90; Found: C, 74.67; H, 8.58; N, 7.85; 1H NMR(500 MHz, CDCl3, ppm) δ 9.71(s, 1H), 7.72(q, 2H), 6.69 (d, J = 8.8 Hz, 2H), 1.24 (t, 6H), 3.46 (q,4H). The second step: The potassium (0.78 g, 0.02 mol) was introduced into tert-butanol (11.3 mL,0.12 mol) in a 50 mL one-necked flask fitted with a condenser. After fully reaction, 4-picoline (0.975mL, 10 mmol) and 4-diethylaminobenzaldehyde (1.77 g, 10 mmol) were added. The mixture washeated for 2 hours at 80°C under stirring; tert-butanol was evaporated, then added to 200 mLdichloromethane and washed once with 200 mL ice water, stirred. The water phase was extracted withdichloromethane for 3 times. The organic phase was dried on sodium sulfate, solvent was evaporated,and the crude solid was recrystallized in toluene. Yield: 86%. 1H NMR (500 MHz, DMSO, ppm) δ8.43 (d, J = 5.2 Hz, 2H), 7.42 (t, J = 8.8 Hz, 2H), 7.23 (d, J = 5.4 Hz, 2H), 7.04 (d, J = 8.7 Hz, 2H),6.27-6.66 (m, 2H), 3.39 (m, 4H), 1.13 (t, 6H). 13C NMR (500 MHz, CDCl3, ppm) δ 148.56-146.96 (m,py), 134.57 (s, py), 128.86 (s, benzene) 120.51-119.95 (d, benzene), 111.56 (s, CH=CH), 44.47 (s,CH2), 12.62 (s, CH3). Anal. Calcd. for C17H20N2 (EI-MS: 253.3): C, 80.91; H, 7.99; N, 11.10. Found: C,80.76; H, 7.91; N, 11.01. IR data (KBr, cm-1): 3026 w, 2974 m, 1581 s, 1520 vs, 1408 m, 1355 m,1329 w, 1272 m, 1185 m, 1158 m, 1074 w, 972 w, 818 m, 795 m, 518 w. m.p. 258 °C.
References:
Li, Dandan;Zhang, Qiong;Wang, Xuchun;Li, Shengli;Zhou, Hongping;Wu, Jieying;Tian, Yupeng [Dyes and Pigments,2015,vol. 120,art. no. 4730,p. 175 - 183] Location in patent:supporting information
![[4-(DIETHYLAMINO)PHENYL]METHANOL](/CAS/GIF/74974-49-5.gif)
74974-49-5
9 suppliers
inquiry

120-21-8
311 suppliers
$5.00/5g


![[(difluoromethyl)thio]benzene](/CAS/GIF/1535-67-7.gif)