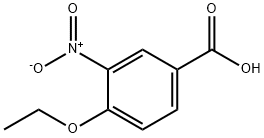
4-Ethoxy-3-nitrobenzoic acid synthesis
- Product Name:4-Ethoxy-3-nitrobenzoic acid
- CAS Number:59719-77-6
- Molecular formula:C9H9NO5
- Molecular Weight:211.17
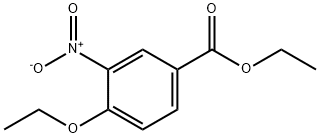
937625-32-6
9 suppliers
inquiry

59719-77-6
42 suppliers
$45.00/10mg
Yield:59719-77-6 96%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;ethanol;water at 20; for 72 h;
Steps:
6 Reference Example 6 4-ethoxy-3-nitrobenzoic acid
DMF (300 ML) was added to 4-hydroxy-3-nitrobenzoic acid (48.16 g, 0.26 mol) and potassium carbonate (128 g, 0.93 mmol). Ethyl iodide (100 mL, 1.25 mol) was added to the obtained suspension and the suspension was stirred at 90°C for 1 hour. Ethyl iodide (50 mL, 0.63 mol) was further added to the reaction solution and the mixture was stirred overnight, after which ethyl acetate and water were added and the mixture was extracted. The obtained organic layer was washed with 1N hydrochloric acid, dried over magnesium sulfate, and purified by silica gel column to give ethyl 4-ethoxy-3-nitrobenzoate. Ethyl 4-ethoxy-3-nitrobenzoate was dissolved in a mixed solvent of THF (300 mL) and ethanol (200 mL). An aqueous solution of 2N sodium hydroxide (300 mL) was added to the obtained solution at room temperature. After stirring the reaction solution at room temperature for 3 days, 6N hydrochloric acid (120 ML) was added, and the solution was concentrated under reduced pressure to remove organic solvents. The resulting precipitates were collected by filtration, washed with water and dried to give the title compound (53.1 g, yield 96%). 1H NMR (300 MHz, CDC13) 8 ppm: 1.52 (t, J = 7.1 Hz, 3 H), 4.28 (q, J = 7.0 Hz, 2 H), 7.14 (d, J = 9.0 Hz, 1 H), 8.25 (dd, J = 8.9, 2.1 Hz, 1 H), 8.55 (d, J = 2.1 Hz, 1 H).
References:
WO2004/46107,2004,A1 Location in patent:Page 92-93
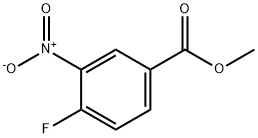
329-59-9
179 suppliers
$8.00/5g

59719-77-6
42 suppliers
$45.00/10mg
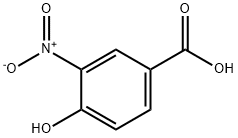
616-82-0
227 suppliers
$10.00/5g

59719-77-6
42 suppliers
$45.00/10mg
25117-74-2
122 suppliers
$13.00/1g

59719-77-6
42 suppliers
$45.00/10mg

619-86-3
241 suppliers
$15.00/25g

59719-77-6
42 suppliers
$45.00/10mg