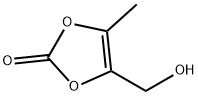
4-(Hydroxymethyl)-5-methyl-1,3-dioxol-2-one synthesis
- Product Name:4-(Hydroxymethyl)-5-methyl-1,3-dioxol-2-one
- CAS Number:91526-18-0
- Molecular formula:C5H6O4
- Molecular Weight:130.1
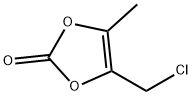
80841-78-7
541 suppliers
$10.00/250mg

91526-18-0
252 suppliers
$6.00/1g
Yield:91526-18-0 79%
Reaction Conditions:
Stage #1:4-chloromethyl-5-methyl-1,3-dioxol-2-one with formic acid;triethylamine in acetonitrile at 10 - 65;
Stage #2: with hydrogenchloride in isopropyl alcoholProduct distribution / selectivity;Reflux;
Steps:
4.A
Example 4APreparation of 4-hydroxymethyl-5-methyl-l, 3-dioxol-2-oneTo a solution of 4-chloromethyl-5methyl-l ,3-dioxol-2-one (50 g) in acetonitrile (500 ml), formic acid (45 g) was added at 20-25°C and the reaction mass cool to 10-15°C followed by addition of triethylamine (95 g). Reaction mass was heated to 60-65 °C and stirred at the same temperature for about 5-6 hrs. Reaction mass was cooled to 15-20°C, filtered and washed by acetonitrile. Filtrate was taken and acetonitrile was distilled out under vacuum to concentrate the solution. Reaction mass was cooled to 25-30°C and ethyl acetate and water was added, followed by stirring for about 15-20 minutes at 25- 30°C. Aqueous layer extracted with ethyl acetate and combined organic layer were washed with brine solution. Organic layer was separated and evaporated completely under vacuum below 35-40°C. To the oily mass, methanol was added and heated to reflux temperature. A solution of HC1 in isopropyl alcohol was added and the resulting solution was refluxed for about 60-75 mins. Reaction mass was cooled to 30-35°C. Distilled out the solvent completely to obtain title compound as oily mass. (Yield: 34.6 g; 79%)
References:
JUBILANT LIFE SCIENCES LIMITED;BANSAL, Deepak;MISHRA, Himanchal;DUBEY, Shailendr, Kumar;CHOUDHARY, Alka, Srivastava;VIR, Dharam;AGARWAL, Ashutosh WO2012/107814, 2012, A1 Location in patent:Page/Page column 14-15
37830-90-3
384 suppliers
$16.00/1g

91526-18-0
252 suppliers
$6.00/1g

91526-17-9
16 suppliers
inquiry

91526-18-0
252 suppliers
$6.00/1g

80715-22-6
97 suppliers
$60.00/50mg

91526-18-0
252 suppliers
$6.00/1g