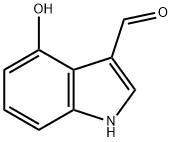
4-HYDROXY-1H-INDOLE-3-CARBALDEHYDE synthesis
- Product Name:4-HYDROXY-1H-INDOLE-3-CARBALDEHYDE
- CAS Number:81779-27-3
- Molecular formula:C9H7NO2
- Molecular Weight:161.16
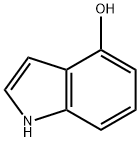
2380-94-1
471 suppliers
$8.00/250mg

81779-27-3
89 suppliers
$43.00/250mg
Yield:-
Reaction Conditions:
with sodium hydroxide;trichlorophosphate in N-methyl-acetamide;water
Steps:
1 4-Hydroxyindole-3-carbaldehyde(1-1) (R7=R11=H)
4-Hydroxyindole-3-carbaldehyde(1-1) (R7=R11=H) Phosphorous oxychloride 7.35 ml was added dropwise to dry dimethylformamide 15 ml under cooling in ice-methanol bath and the mixture was stirred for 15 min. Then, a solution of the 4-hydroxyindole 5.0 g in dry dimethylformamide 10 ml was added dropwise to the mixture under cooling in ice and the mixture was stirred for 2 h at room temperature. Water was added under cooling in ice to the mixture, which was made alkaline with a 30% aqueous sodium hydroxide solution and was stirred for 15 min. Then, the mixture was acidified to pH 4 with 5N-HCl and the precipitate was collected by filtration, washed with water and dried to give the titled compound 4.99 g as crude crystalline materials. Yield 82%. Crude crystalline materials are recrystallized from methanol to give yellow crystals m.p. 190-193 ØC. 1H-NMR(DMSO-d6): 6.54 (1H, dd, J = 8.1, 0.9 Hz), 6.95 (1H, dd, J = 8.1, 0.9 Hz), 7.13 (1H, t, J = 8.1 Hz), 8.37 (1H, s), 9.64 (1H, s), 10.54 (1H, br s), 12.35 (1H, br s).
References:
SHIONOGI & CO., LTD. EP1325909, 2003, A1
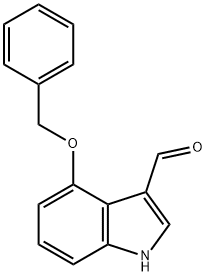
7042-71-9
126 suppliers
$41.00/100mg

81779-27-3
89 suppliers
$43.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

81779-27-3
89 suppliers
$43.00/250mg
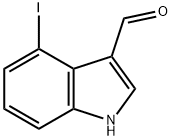
72527-73-2
11 suppliers
inquiry

81779-27-3
89 suppliers
$43.00/250mg
487-89-8
601 suppliers
$6.00/10g

81779-27-3
89 suppliers
$43.00/250mg