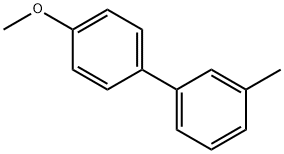
4-METHOXY-3'-METHYLBIPHENYL synthesis
- Product Name:4-METHOXY-3'-METHYLBIPHENYL
- CAS Number:17171-17-4
- Molecular formula:C14H14O
- Molecular Weight:198.26
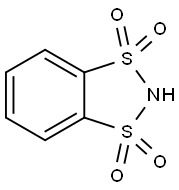
4482-01-3
28 suppliers
$60.00/100mg
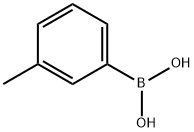
17933-03-8
364 suppliers
$5.00/1g
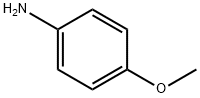
104-94-9
492 suppliers
$9.00/1g

17171-17-4
24 suppliers
$437.00/1g
Yield: 79%
Reaction Conditions:
Stage #1:o-benzenedisulfonimide;4-methoxy-aniline with acetic acid at 0 - 5; for 0.166667 h;Inert atmosphere;
Stage #2: with isopentyl nitrite for 0.166667 h;Inert atmosphere;
Stage #3:m-tolylboronic acid with bis[(trifluoromethanesulfonyl)imidate]-2-(dicyclohexyl(2’,6’-dimethoxybiphenyl))phosphine gold(I);caesium carbonate in tetrahydrofuran at 20; for 3 h;Inert atmosphere;chemoselective reaction;Suzuki-Miyaura Coupling;
Steps:
3.12 4.3 4-Methoxybiphenyl (4a): representative procedure for the Au catalysed Suzuki-Miyaura couplings
General procedure: In a oven-dried flask and under nitrogen flow, benzenediazonium o-benzenedisulfonimide (1a, 161mg, 0.5mmol) was added to a suspension of 4-methoxyphenylboronic acid (3a, 91mg, 0.6mmol), [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold(I) (2:1) toluene adduct (39mg, 0.025mmol, 5mol%), Cs2CO3 (325mg, 1mmol) in THF (5mL). The resulting mixture was stirred at room temperature for 2h; the completion of the reaction was confirmed by the absence of azo coupling with 2-naphthol. Then, the reaction mixture was poured into diethyl ether/water (100mL, 1:1). The aqueous layer was separated and extracted with diethyl ether (50mL). The combined organic extracts were washed with water (50mL), dried with Na2SO4 and evaporated under reduced pressure. GC-MS analyses of the crude residue showed 4-methoxybiphenyl (4a), MS (EI): m/z 184 (M+) as the major product, besides traces of biphenyl, MS (EI): m/z 154 (M+), 4,4'-dimethoxybiphenyl, MS (EI): m/z 214 (M+), N-phenyl-o-benzenedisulfonimide, MS (EI): m/z 295 (M+). The crude residue was purified on a short column, eluting with petroleum ether/diethyl ether (9:1). The only isolated product was the title compound (4a, 81mg, 88% yield).
References:
Barbero, Margherita;Dughera, Stefano [Tetrahedron,2018,vol. 74,# 39,p. 5758 - 5769]
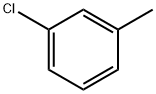
108-41-8
266 suppliers
$19.00/10g
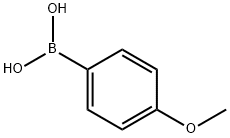
5720-07-0
470 suppliers
$6.00/5g

17171-17-4
24 suppliers
$437.00/1g
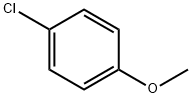
623-12-1
285 suppliers
$10.00/25g

17933-03-8
364 suppliers
$5.00/1g

17171-17-4
24 suppliers
$437.00/1g
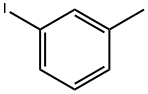
625-95-6
251 suppliers
$14.00/25g

5720-07-0
470 suppliers
$6.00/5g

17171-17-4
24 suppliers
$437.00/1g

108-41-8
266 suppliers
$19.00/10g

13139-86-1
135 suppliers
$45.00/5g

17171-17-4
24 suppliers
$437.00/1g