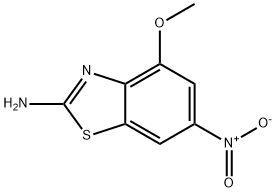
4-METHOXY-6-NITRO-BENZOTHIAZOL-2-YLAMINE synthesis
- Product Name:4-METHOXY-6-NITRO-BENZOTHIAZOL-2-YLAMINE
- CAS Number:16586-52-0
- Molecular formula:C8H7N3O3S
- Molecular Weight:225.22
Yield: 58%
Reaction Conditions:
with bromine;acetic acid at 5 - 95; for 16.5 h;
Steps:
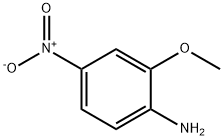
4-Methoxy-6-nitrobenzo[d]thiazol-2-amine (159-2)
At 5° C., a solution of bromine (5.57 mL, 110 mmol, 1.1 eq) in acetic acid (50 mL) was added slowly to an acetic acid solution (200 mL) of 2-methoxy-4-methylaniline (16.8 g, 100 mmol) and ammonium thiocyanate (11.4 g, 150 mmol, 1.5 eq) under vigorous stirring.
After the completion of addition, the reaction mixture was allowed to warm to 25° C. and stirred for 16 hours.
Then the reaction mixture was heated to 95° C. and stirred at this temperature for 30 minutes.
After the completion monitored by silica gel TLC, the reaction mixture was poured into ice water and basified to pH=11 with ammonium hydroxide (m %=28-30% aqueous solution) under vigorous stirring.
Then the suspension was stirred at room temperature for 30 minutes.
After filtration, the solid obtained was stirred in methanol/ethyl acetate (v/v=1/1, 200 mL) at room temperature for 1 hour.
Then, filtration gave the product (13 g, yield=58%) as a yellow solid. 1H NMR (600 MHz, DMSO-d6): δ 8.38 (d, J=2.2 Hz, 1H), 8.15 (s, 2H), 7.64 (d, J=2.1 Hz, 1H), 3.95 (s, 3H). LRMS m/z 226.0 [M+H]+.
References:
The Regents of the University of Michigan;Cierpicki, Tomasz;Grembecka, Jolanta;Huang, Huang;Zari, Sergi;Cho, Hyo Je;Potopnyk, Mykhaylo;Dudkin, Sergeii;Chen, Wenbing;Adam, Yassir;Howard, Christina;Kim, EunGi US2019/183865, 2019, A1 Location in patent:Paragraph 0412; 0413
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)