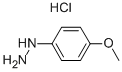
4-Methoxyphenylhydrazine hydrochloride synthesis
- Product Name:4-Methoxyphenylhydrazine hydrochloride
- CAS Number:19501-58-7
- Molecular formula:C7H11ClN2O
- Molecular Weight:174.63
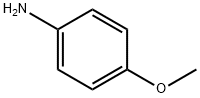
104-94-9
473 suppliers
$9.00/1g

19501-58-7
368 suppliers
$7.00/250mg
Yield:19501-58-7 77%
Reaction Conditions:
Stage #1: 4-methoxy-anilinewith hydrogenchloride;sodium nitrite in water; for 1.75 h;Cooling;
Stage #2: with hydrogenchloride;tin(ll) chloride in water at 0; for 0.5 h;
Steps:
(4-Methoxyphenyl)hydrazine (4)
To asolution of p-anisidine (4.93 g, 40mmol) in water (12 mL) and conc. HCl (12 mL) was added a solution of sodiumnitrite (3.3 g, 46.40 mmol) in water (10 mL) dropwise at -5 oC overthe period of 15 min. The reaction mixture was stirred for 1.5 h and a solutionof SnCl2 (16.00 g, 84.40 mmol) in conc. HCl (25 mL) was addeddropwise and stirred manually in spite of bulky precipitation. After thereaction mixture was stirred at 0 oC for 30 min, the precipitate wascollected by filtration, washed with water (20 mL), ethanol (12 mL), and Et2O(50 mL) and dried in vacuo to give compound 4 (5.40 g, 31.00 mmol, 77%) as a pink solid: Rf =0.23 (n-hexane : EtOAc = 2:1); 1H NMR (400MHz, D2O) δ6.94 (2H, d, J = 8.8 Hz), 6.84 (2H, d, J = 8.8Hz), 3.64 (3H, s).
References:
Gim, Hyo Jin;Li, Hua;Jeong, Ji Hye;Lee, Su Jeong;Sung, Mi-Kyung;Song, Mi-Young;Park, Byung-Hyun;Oh, Soo Jin;Ryu, Jae-Ha;Jeon, Raok [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 13,art. no. 12258,p. 3322 - 3336] Location in patent:supporting information
4346-59-2
3 suppliers
inquiry

19501-58-7
368 suppliers
$7.00/250mg
1352877-26-9
0 suppliers
inquiry

19501-58-7
368 suppliers
$7.00/250mg
696-62-8
349 suppliers
$5.00/1g

19501-58-7
368 suppliers
$7.00/250mg
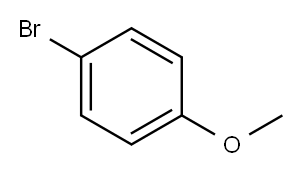
104-92-7
424 suppliers
$10.00/10g

19501-58-7
368 suppliers
$7.00/250mg