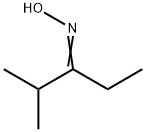
4-METHYL-2-PENTANONE OXIME synthesis
- Product Name:4-METHYL-2-PENTANONE OXIME
- CAS Number:105-44-2
- Molecular formula:C6H13NO
- Molecular Weight:115.17

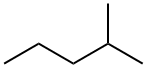
108-10-1
895 suppliers
$12.00/25ml

105-44-2
107 suppliers
$60.00/500mg
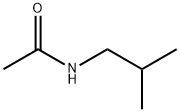
1540-94-9
7 suppliers
inquiry
Yield: 74% , 8%
Reaction Conditions:
with acetylhydroxamic acid;sulfuric acid in acetonitrile for 11.5 h;Reflux;
Steps:
Typical procedure for the preparation of amide under conventional heating:
General procedure: Acetophenone 1a (1.0 g, 8.3 mmol), acetohydroxamic acid (0.92 g, 12.5 mmol), acetonitrile (10 ml), and conc. H2SO4 (0.2 ml) were taken into a 25 ml round bottomed flask fitted with a condenser and calcium chloride guard tube. The mixture was refluxed and the progress of the reaction was monitored by tlc. After completion of the reaction (8.5 h), the reaction mixture was cooled to room temperature and diluted with ethyl acetate (5 ml). Next, saturated sodium bicarbonate solution (10 ml) was added drop-wise to the mixture for the neutralization of sulfuric acid and then extracted with ethyl acetate (2 × 10 ml). The combined organic layer was washed with saturated NaCl solution, dried over anhy. Na2SO4, and concentrated under reduced pressure. The crude product was purified by normal column chromatography (silica gel 60-120 mesh, ethyl acetate/hexane: 1:4) to obtain acetanilide 2a (0.89 g, 80%, mp 114-116 °C) and acetophenone oxime 3a (76 mg, 7%, mp 55-60 °C) in the form of white powders.
References:
Madabhushi, Sridhar;Chinthala, Narsaiah;Vangipuram, Venkata Sairam;Godala, Kondal Reddy;Jillella, Raveendra;Mallu, Kishore Kumar Reddy;Beeram, China Ramanaiah [Tetrahedron Letters,2011,vol. 52,# 46,p. 6103 - 6107] Location in patent:experimental part

108-10-1
895 suppliers
$12.00/25ml

105-44-2
107 suppliers
$60.00/500mg