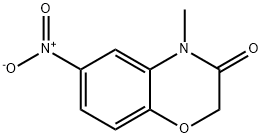
4-Methyl-6-nitro-2H-1,4-benzoxazin-3-one synthesis
- Product Name:4-Methyl-6-nitro-2H-1,4-benzoxazin-3-one
- CAS Number:103361-68-8
- Molecular formula:C9H8N2O4
- Molecular Weight:208.17

81721-87-1
80 suppliers
$16.00/500mg

74-88-4
349 suppliers
$15.00/10g

103361-68-8
12 suppliers
inquiry
Yield:103361-68-8 100%
Reaction Conditions:
Stage #1: 6-nitro-4H-benzo[1,4]oxazin-3-onewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.166667 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 0; for 1 h;
Steps:
8.2
Step 2; 4-Methyl-6-nitro-4H-benzo[1,4]oxazin-3-oneNaH (0.57 g, 23.75 mmol) was added to a solution of 6-nitro-4H-benzo[1,4]oxazin-3-one (2.3 g, 11.85 mmol) in DMF (50 ml) at 0° C. The mixture was stirred at 0° C. for 10 minutes and then CH3I (1.5 ml, 24.04 mmol) was added. The mixture was stirred at 0° C. for one hour, then at room temperature for one hour. The mixture was partitioned between EtOAc and water. The organic layer was dried (MgSO4), filtered, and concentrated under reduced pressure to give 2.5 g of 4-methyl-6-nitro-4H-benzo[1,4]oxazin-3-one as a solid, 100%.
References:
US2010/160373,2010,A1 Location in patent:Page/Page column 30

21744-84-3
86 suppliers
$13.43/250mgs:

103361-68-8
12 suppliers
inquiry

81721-87-1
80 suppliers
$16.00/500mg

103361-68-8
12 suppliers
inquiry

99-57-0
9 suppliers
$22.00/25g

103361-68-8
12 suppliers
inquiry
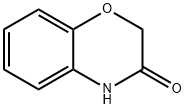
5466-88-6
190 suppliers
$23.00/5g

103361-68-8
12 suppliers
inquiry