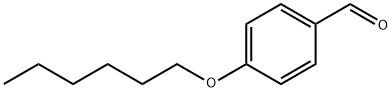
4-N-HEXYLOXYBENZALDEHYDE synthesis
- Product Name:4-N-HEXYLOXYBENZALDEHYDE
- CAS Number:5736-94-7
- Molecular formula:C13H18O2
- Molecular Weight:206.28
Yield:5736-94-7 98%
Reaction Conditions:
with potassium carbonate in acetonitrile;Reflux;Inert atmosphere;
Steps:
I.1
1) 4-Hexyloxybenzaldehyde, Intermediate Compound 14-formyl-phenol (5 g, 41 mmol), iodohexane (10.5 g, 49 mmol) and K2CO3 (8.5 g, 61 mmol) in acetonitrile (150 ml) were refluxed overnight under N2. After being cooled to room temperature, water (10 ml) was added and acetonitrile was evaporated. Water (150 ml) and Et2O (150 ml) were then added. The ethereal layer was extracted and washed with water (2×100 ml), brine (100 ml), dried over MgSO4, filtered and evaporated to dryness to afford 8.3 g (98%) of compound 1 as a slightly yellow oil after during at 80° C. under vacuum.1H-NMR (CDCl3, 298K, 200 MHz, δ ppm) 0.94 (t, J=6.5 Hz, 3H), 1.3-1.6 (m, 6H), 1.80 (m, 2H), 4.05 (t, J=6.5 Hz, 2H), 7.00 (d, J=8.7 Hz, 2H), 7.83 (d, J=8.7 Hz, 2H), 9.89 (s, 1H). 13C-NMR (CDCl3, 298K, 50 MHz, δ ppm) 14.0, 22.5, 25.6, 29.0, 31.5, 68.4, 114.7, 129.7, 131.9, 164.2, 190.7.
References:
US7932404,2011,B2 Location in patent:Page/Page column 17
57792-40-2
3 suppliers
inquiry

5736-94-7
111 suppliers
$39.59/5g
1122-91-4
673 suppliers
$9.00/10g

111-27-3
497 suppliers
$5.00/25g

5736-94-7
111 suppliers
$39.59/5g



