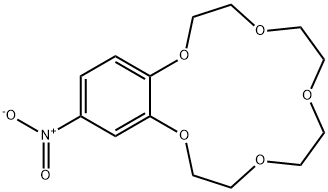
4-NITROBENZO-15-CROWN-5 synthesis
- Product Name:4-NITROBENZO-15-CROWN-5
- CAS Number:60835-69-0
- Molecular formula:C14H19NO7
- Molecular Weight:313.3
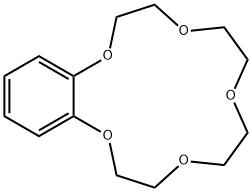
14098-44-3
211 suppliers
$28.00/1g

60835-69-0
106 suppliers
$35.29/1g
Yield:60835-69-0 99.5%
Reaction Conditions:
with nitric acid in acetonitrile
Steps:
4-Nitrobenzo-15-crown-5 production (NB15C5)
Benzo-15-crown-5 nitration was carried outby nitric acid, 58%, over boiling reaction mass in theacetonitrile media, using relevant method17.Nitric acid of various concentrations can beused in nitration reaction. In some cases, diluted nitric acid (which is poor nitration agent and quite strongoxidizing agent) is also used due to the constantpresence of nitrous acid that hinders nitration.Another drawback of diluted nitric acid, which limitsits use, is its ability to express nitrating effect only athigh temperatures, which are featured by prevailingside oxidation reaction. Concentrated nitric acidshows nitrating effect at lower temperatures and lesslikely to cause undesirable oxidation, which is why wecarried out a nitration of resulted benzo-15-crown-5with nitric acid, 56%, in the acetonitrile media. Theyield of target product was 99.5%, while the meltingpoint of resulted compound was 94-95 0C.Infrared spectrum (KBr, ν, cm-1): 497(weak), 538 (weak), 618 (weak), 654 (medium)(aromatic C-H), 723 (weak), 745 (medium) (NO2),787 (weak), 806 (medium), 868 (medium) (N-O), 913(weak), 934 (medium), 980 (medium), 1010(weak),1047 (medium), 1093 (strong) (C-N), 1138 (strong)(C-O-C), 1240 (strong) (NO2), 1276 (strong) (NO2),1335 (strong) (NO2), 1417 (strong), 1428 (strong),1448 (medium) (N=O), 1517 (strong) (CH2 + NO2),1587 (medium) (aromatic C-C), 2868 (medium)(C-H), 2904 (medium) (C-H), 2929 (medium) (C-H),3083 (medium) (aromatic C-H).1H N u c l e a r m a g n e t i c r e s o n a n c e(DMSO-d6, 300 MHz): 3.62 (broad singlet, 8H,CH2+CH2+CH2+CH2), 3.74-3.85 (multiplet, 4H,CH2+CH2), 4.13-4.24 (multiplet, 4H, CH2+CH2), 7.15(doublet, 1H, J=8.9, CH), 7.73 (doublet, 1H, J=2.7,CH), 7.89 (double doublet, 1H, J=8.9, J=2.7, CH).13C Nuclear magnetic resonance (DMSO-d6,75 MHz): 67.77, 68.84, 69.27, 69.71, 70.09, 70.16,70.30, 100.96, 105.53, 117.49, 139.35, 143.95,149.92.
References:
Glushko, Valentina N.;Sadovskaya, Natalya Yu.;Kozhuhov, Vadim I.;Blokhina, Lidiya I.;Antropova, Irina A.;Petina, Ekaterina S.;Retiviov, Vasiliy M.;Melnikova, Ekaterina Yu. [Oriental Journal of Chemistry,2017,vol. 33,# 4,p. 1689 - 1697]
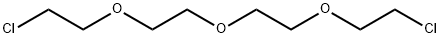
638-56-2
83 suppliers
$26.00/5g

60835-69-0
106 suppliers
$35.29/1g
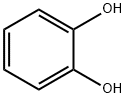
120-80-9
496 suppliers
$13.00/25g

60835-69-0
106 suppliers
$35.29/1g