
4-NITROCATECHOL synthesis
- Product Name:4-NITROCATECHOL
- CAS Number:3316-09-4
- Molecular formula:C6H5NO4
- Molecular Weight:155.11
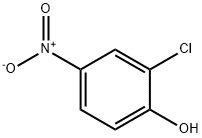
619-08-9
316 suppliers
$5.00/1g

3316-09-4
164 suppliers
$6.00/1g
Yield:3316-09-4 84%
Reaction Conditions:
with sodium hydroxide in water for 6 h;Heating / reflux;
Steps:
2
p-nitrophenol (69.55 g, 0.5 mole) was dissolved in 10 N HCl (200 ml) in a 1000 ml flask with heating and then cooled down. To the mixture was added 600 ml potassium chlorate (61.25 g, 0.5 mole) solution, gradually with stirring. Stirring was continued for 1 hour after completion of addition and mixture was left overnight at room temperature. After filtration with vacuuming, the filtrate was washed with distilled water and recrystallized with dilute acetic acid solution to yield pale yellow acicular crystal, 2-chloro4-nitro-phenol (80.2 g, m.p. 110-111° C., yield rate 91%).2-chloro-4-nitro-phenol (64.44 g, 0.4 mole) and 300 ml 4M NaOH solution were refluxed with heating for 6 hours. After cooling down and filtration with vacuuming, the filtrate (crystal) was dissolved in a little warm water (60° C.). The solution was adjusted to pH 2.0 with 10% HCl and cooled down while stirring. After filtration, the filtrate was recrystallized with water to yield 4-nitro-1,2-benzenediol (52.1 g, yield rate 84%).To a 1000 ml flask was added 4-nitro-1,2-benzenediol (49.6 g, 0.32 mole), and then 403 ml water and 16 ml 10 N HCl (0.16 mole). While stirring 83.2 g zinc dust (1.28 mole) was added gradually and reaction mixture refluxed with heating for 8 hours. After cooling down and filtration with vacuuming, the filtrate was washed thoroughly with 95% ethanol and decolorized by active carbon. Filtration again and evaporation of ethanol was carried out until the solution just started to become turbid. After cooling in freezer and filtration with vacuuming, filtrate was collected as 4-amino-1,2-benzenediol (30.0 g, yield rate 75%).Dissolve 4-amino-1,2-benzenediol (23.2 g, 0.18 mole) in 95% ethanol. Under salt ice bath 140 ml 2M NaOH solution and 150 ml dichloromethane solution of 13-methyltetradecanoyl chloride (23.2 g, 0.18 mole) were added simultaneously to the benzenediol solution. Stir at room temperature for 6 hours. Adjust pH to 3.0 with HCl, freeze, and filtrate. Wash the filtrate to neutral with water and recrystallize with 50% ethanol to yield N-(3,4dihydroxyl-phenyl)-13-methyl-tetradecanamide (55.8 g, m.p. 85-86° C., yield rate 85%).
References:
Yang, Zhenhua US7109364, 2006, B2 Location in patent:Page/Page column 23-24
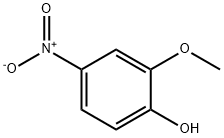
3251-56-7
205 suppliers
$8.00/1g

3316-09-4
164 suppliers
$6.00/1g
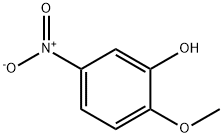
636-93-1
265 suppliers
$6.00/5g

3316-09-4
164 suppliers
$6.00/1g

97-51-8
416 suppliers
$6.00/5g

3316-09-4
164 suppliers
$6.00/1g
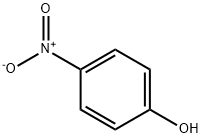
100-02-7
5 suppliers
$11.00/5G

3316-09-4
164 suppliers
$6.00/1g
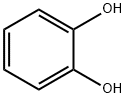
120-80-9
496 suppliers
$13.00/25g
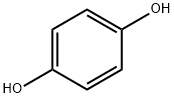
123-31-9
825 suppliers
$13.00/25g
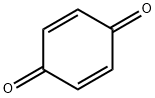
106-51-4
479 suppliers
$10.00/5g