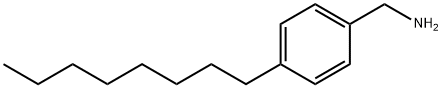
4-OCTYLBENZYLAMINE synthesis
- Product Name:4-OCTYLBENZYLAMINE
- CAS Number:176956-02-8
- Molecular formula:C15H25N
- Molecular Weight:219.37
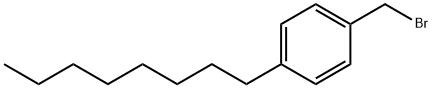
88255-11-2
12 suppliers
inquiry

176956-02-8
17 suppliers
inquiry
Yield:176956-02-8 100%
Reaction Conditions:
with sodium hexamethyldisilazane;1,1,1,3,3,3-hexamethyl-disilazane in tetrahydrofuran at 20;Inert atmosphere;
Steps:
31.D
To a solution of the product of Step C (0.13 g; 0.459 mmol) in anhydrous hexamethylenedisilazane (HMDSA) 1 M NaHMDSA in THF was added at room temperature under N2 with stirring. After stirring overnight at room temperature the solvents were removed under reduced pressure and the residue was diluted to 5 ml with MeOH and 1 drop of concentrated HCI was added. This was evaporated under reduced pressure, diluted to 15 ml with Et2O and washed with 1 N NaOH, brine, dried over anhydrous MgSO4 and filtered. The filtrate was evaporated under reduced pressure to give the title compound (0.1 g; 100%), as colourless oil, which was used in the next Step without further purification. . 1 H-NMR (CDCI3) 0.86 (tr, 3H, J = 7 Hz); 1 .26 (m, 10H); 1 .41 (s, 2H); 1 .58 (m, 2H); 2.57 (tr, 2H, J = 7.9 Hz); 3.82 (s, 2H); 7.13 (d, 2H, J = 8 Hz); 7.2 (d, 2H, J = 8 Hz).
References:
WO2010/42998,2010,A1 Location in patent:Page/Page column 88
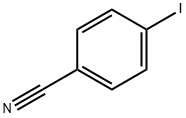
3058-39-7
278 suppliers
$10.00/1g

176956-02-8
17 suppliers
inquiry

111-66-0
145 suppliers
$10.00/10ml

176956-02-8
17 suppliers
inquiry
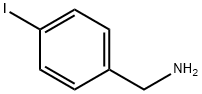
39959-59-6
124 suppliers
$5.00/100mg

176956-02-8
17 suppliers
inquiry

629-05-0
167 suppliers
$10.00/1g

176956-02-8
17 suppliers
inquiry