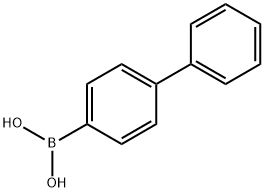
4-Phenyl-1,1':2',1''-terbenzene synthesis
- Product Name:4-Phenyl-1,1':2',1''-terbenzene
- CAS Number:1165-58-8
- Molecular formula:
- Molecular Weight:0
Yield:1165-58-8 90%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in water;N,N-dimethyl-formamide at 0; for 5 h;Inert atmosphere;Suzuki-Miyaura Coupling;
Steps:
Suzuki-Miyaura coupling to form o,p’-quaterphenyl (15)[35]:
K2CO3 (0.91 g, 6.6 mmol) and water (7.7 mL) werecombined in a 25 mL round bottom flask which was purgedwith nitrogen, and cooled to 0 °C. Biphenyl-4-boronic acid(0.89 g, 4.5 mmol) was added, followed by 2-bromobiphenyl(0.50 g, 2.2 mmol), DMF (11.5 mL) and Pd(PPh3)4 (0.13 g,0.1 mmol). The reaction mixture was stirred at 0 °C for 5 h.NaOH (1 M, 10 mL) was added and the product was extracted with DCM. The organic extract was washed with water andbrine and dried with Na2SO4. The crude product was purifiedby flash chromatography with hexanes to yield o,p’-quaterphenylas a white solid (0.55 g, mp 107-109 °C, lit 117-120 °C,90% yield). 1H NMR (400 MHz, CDCl3) δ 7.61-7.57 (m, 2H),7.50-7.40 (m, 8H), 7.35-7.29 (m, 1H), 7.26-7.17 (m, 7H).
References:
Skraba-Joiner, Sarah L.;Holt, Carter J.;Johnson, Richard P. [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 2655 - 2663]
1444-65-1
143 suppliers
$12.00/250mg

1165-58-8
2 suppliers
inquiry
1591-31-7
337 suppliers
$8.00/5g

1165-58-8
2 suppliers
inquiry
92-66-0
567 suppliers
$10.00/1g

1165-58-8
2 suppliers
inquiry
2436-96-6
143 suppliers
$55.00/25g

1165-58-8
2 suppliers
inquiry