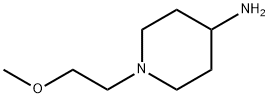
4-Piperidinamine,1-(2-methoxyethyl)-(9CI) synthesis
- Product Name:4-Piperidinamine,1-(2-methoxyethyl)-(9CI)
- CAS Number:502639-08-9
- Molecular formula:C8H18N2O
- Molecular Weight:158.24
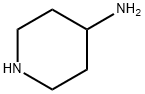
13035-19-3
12 suppliers
$9.00/1g
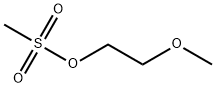
16427-44-4
96 suppliers
$15.00/1g

502639-08-9
24 suppliers
$50.00/250mg
Yield:502639-08-9 9.5%
Reaction Conditions:
Stage #1: 4-aminopiperidinewith sodium carbonate for 4 h;Reflux;
Stage #2: 2-methoxyethyl methanesulfonate at 22 - 25; for 16 h;
Steps:
27.1 Step I : Preparation of l -(2-methoxyethyl)piperidin-4-amine
4-amino piperidine (2 g. 19.96 mmol) and anhydrous sodium carbonate (5.29 g, 49.9 mmol) was dissolved in 25 mL of methyl isobutyl ketone. The heterogeneous reaction mass was heated to reflux, and maintained for 4 h. 2-Methoxyethyl methanesulfonate (2.77 g, 17.96 mmol) was added to the above mixture and stirred for 16 h at 22 to 25 °C. The reaction mass was filtered and 25 mL of IN aq.HCl was added and stirred for another 1 h. The aqueous phase was separated, and pH was adjusted to 1 1.0 using IN aq. NaOH. Compound was extracted into DCM, dried over anhydrous sodium sulfate and cone under vacuum to get 1- (2-methoxyethyl)piperidin-4-amine ((300 mg, 9.5% yield)
References:
WO2015/79459,2015,A1 Location in patent:Paragraph 00311
502639-07-8
8 suppliers
$370.00/1g

502639-08-9
24 suppliers
$50.00/250mg
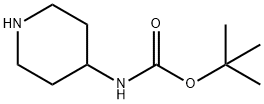
73874-95-0
446 suppliers
$10.00/250mg

502639-08-9
24 suppliers
$50.00/250mg