
4-Piperidinecarboxamide,1-acetyl- synthesis
- Product Name:4-Piperidinecarboxamide,1-acetyl-
- CAS Number:530120-27-5
- Molecular formula:C8H14N2O2
- Molecular Weight:170.21
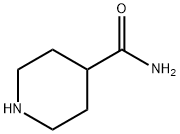
39546-32-2
345 suppliers
$6.00/5g

108-24-7
0 suppliers
$14.00/250ML

530120-27-5
23 suppliers
$45.00/10mg
Yield:530120-27-5 100%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 18 h;Product distribution / selectivity;
Steps:
3
EXAMPLE 3; Synthesis of l-acetylpiperidine-4-isocyanate; To a solution of piperidine-4-carboxamide (6.0 mmol) in CH2Cl2 (30 mL) was addedEt3N (2.5 mL, 18.0 mmol) followed by acetic anhydride (0.7 mL, 7.2 mmol, 1.2 equiv.) at 0-5 0C. The reaction mixture was allowed to warm to room temperature and was stirred at ambient for 18 hours. The precipitated solid was collected by filtration, washed with CH2Cl2 (2 x 25 mL), and dried to afford l-acetylpiperidine-4-carboxamide as a white solid in quantitative yield. LCMS 171 [M+H], 1H NMR (300 MHz, CDCl3) δ: 4.53-4.49 (m,IH), 3.98-3.93 (m, IH), 3.19-3.09 (m, IH), 2.73-2.63 (m, IH), 2.54-2.42 (m, IH), 1.89-1.80 (m, 2H), 1.71-1.47 (m, 2H).
References:
WO2008/94862,2008,A1 Location in patent:Page/Page column 37
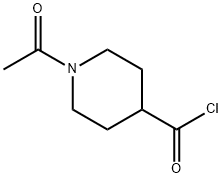
59084-16-1
114 suppliers
$14.14/250mgs:

530120-27-5
23 suppliers
$45.00/10mg