
(4'-TRIFLUOROMETHYL-BIPHENYL-2-YL)-METHANOL synthesis
- Product Name:(4'-TRIFLUOROMETHYL-BIPHENYL-2-YL)-METHANOL
- CAS Number:773871-77-5
- Molecular formula:C14H11F3O
- Molecular Weight:252.23
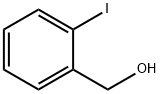
5159-41-1
214 suppliers
$8.00/5g
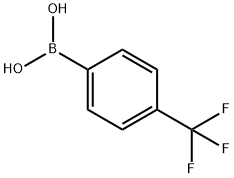
128796-39-4
427 suppliers
$10.00/5g

773871-77-5
12 suppliers
$370.00/250mg
Yield:773871-77-5 99%
Reaction Conditions:
with C22H24Cl2N6O2Pd2;potassium carbonate in ethanol;water; for 4 h;Catalytic behavior;Reflux;Suzuki-Miyaura Coupling;
Steps:
General Procedure for the Suzuki-Miyaura cross-coupling reaction:
General procedure: In a 10 mL glass tube containing a Teflon-coated stir bar was placed p-bromobenzaldehyde 2e (0.05 g, 0.27 mmol, 1 equiv), phenylboronic acid 1a (0.05 g, 0.40 mmol, 1.5 equiv), 2M K2CO3 (0.33 mL, 0.67 mmol, 2.5 equiv), 4-AAP-Pd(II) (0.28 mg, 0.3 mol % Pd) and EtOH (2 mL). The mixture was stirred at reflux for 4 h. After cooling, the mixture was diluted with ether Et2O (5 mL), washed with sat. aq. NaHCO3 (3 mL), brine (3 mL) and dried over Na2SO4. Evaporation of the solvent and purification of the residue over a silica gel column (Hex: AcOEt 90:10), furnished the biphenyl 3q.
References:
Contreras-Celedn, Claudia A.;Mendoza-Rayo, Daro;Rincn-Medina, Jos A.;Chacn-Garca, Luis [Beilstein Journal of Organic Chemistry,2014,vol. 10,p. 2821 - 2826] Location in patent:supporting information

84392-17-6
158 suppliers
$6.00/250mg

773871-77-5
12 suppliers
$370.00/250mg
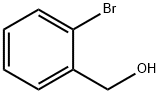
18982-54-2
277 suppliers
$5.00/5g

128796-39-4
427 suppliers
$10.00/5g

773871-77-5
12 suppliers
$370.00/250mg
![2-[4-(TRIFLUOROMETHYL)PHENYL]BENZALDEHYDE](/CAS/GIF/84392-23-4.gif)
84392-23-4
63 suppliers
$45.00/25mg

773871-77-5
12 suppliers
$370.00/250mg

128796-39-4
427 suppliers
$10.00/5g

773871-77-5
12 suppliers
$370.00/250mg