
4-(Triisopropylsilyloxy)phenyl Boronic Acid synthesis
- Product Name:4-(Triisopropylsilyloxy)phenyl Boronic Acid
- CAS Number:643090-93-1
- Molecular formula:C15H27BO3Si
- Molecular Weight:294
Yield:643090-93-1 96%
Reaction Conditions:
Stage #1: 1-iodo-4-[[tris(1-methylethyl)silyl]oxy]benzenewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2: Triisopropyl borate in tetrahydrofuran;hexane at -78; for 80 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;
Steps:
MM-1-188
(MM-1-188) TIPSO iodide MM-1-58 (700 1.86 mmol) was dissolved in anhydrous THF (9 mL) and cooled to -78 °C. n-BuLi (0.89 mL, 2.23 mmol, 2.5 M in hexanes) was added dropwise. After 30 minutes, triisopropyl borate (0.86 mL, 3.72 mmol, 2.00 equiv) was added. After an additional 20 minutes, the cold bath was removed, and the mixture was stirred for 1 hours. 2 N HC1 (10 mL) was added slowly, and the mixture was diluted and extracted with EtOAc (2 χ 10 mL) . The combined organic phases were washed with sat. aqueous NaCl, dried over Na2S04, decanted and concentrated. Flash chromatography (Si02, 40% EtOAc/hexanes) gave 523 mg (96%) of the aryl boronic acid as a white solid.
References:
WO2014/131023,2014,A1 Location in patent:Page/Page column 120-121
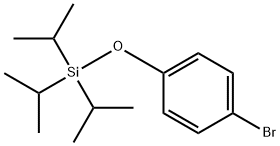
193966-77-7
16 suppliers
$63.50/1g

643090-93-1
7 suppliers
inquiry
![Benzene, 1-iodo-4-[[tris(1-methylethyl)silyl]oxy]-](/CAS/20210111/GIF/180577-83-7.gif)
180577-83-7
1 suppliers
inquiry

643090-93-1
7 suppliers
inquiry
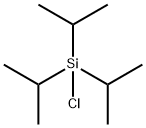
13154-24-0
415 suppliers
$20.00/10g

643090-93-1
7 suppliers
inquiry
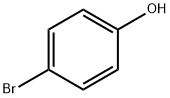
106-41-2
484 suppliers
$13.00/5g

643090-93-1
7 suppliers
inquiry
