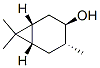
[1R-(1alpha,3alpha,4beta,6alpha)]-4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol synthesis
- Product Name:[1R-(1alpha,3alpha,4beta,6alpha)]-4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol
- CAS Number:4017-88-3
- Molecular formula:C10H18O
- Molecular Weight:154.2493

498-15-7
77 suppliers
$13.98/25ML
![[1R-(1alpha,3alpha,4beta,6alpha)]-4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol](/CAS/GIF/4017-88-3.gif)
4017-88-3
2 suppliers
inquiry
Yield:4017-88-3 78%
Reaction Conditions:
Stage #1: (+)-Δ3-carenewith sodium tetrahydroborate;boron trifluoride diethyl etherate in tetrahydrofuran at 0; for 4.5 h;Inert atmosphere;
Stage #2: with sodium hydroxide in tetrahydrofuran;water at -10; for 0.666667 h;Inert atmosphere;
Stage #3: with dihydrogen peroxide in tetrahydrofuran;water at -10 - 20; for 1 h;Inert atmosphere;
Steps:
(1R,3R,4R,6S)-4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol (S0)
The procedure was adapted from theliterature:2 To a dried 500 mL round bottom flask was added NaBH4 (6.94 g, 183 mmol, 1.0 equiv), THF(60 mL), and (+)-3-carene (25 g, 180 mmol, 1.0 equiv). The reaction flask was cooled to 0 °C in an icebath, and BF3?OEt2 (26 g, 160 mmol, 1.0 equiv) was added dropwise over 30 min. The reaction was stirredat this temperature for 4 h, after which the reaction was cooled to -10 °C and aq. NaOH (3M, 60 mL) wasadded dropwise over 40 min. H2O2 (30 wt %, 100 mL) was then added dropwise over 1 h, and the reactionallowed to warm to 20 °C. The solution was concentrated under reduced pressure to remove THF, and theaqueous layer was extracted with CH2Cl2 (3 x 40 mL). The combined organic layers were washed withwater (100 mL) and brine (100 mL), dried over Na2SO4, and filtered. Concentration under reduced pressureafforded an oil that was distilled under vacuum (0.5 mmHg, 150 °C) to afford S0 (22 g, 78%) as a colorlessoil that solidified upon cooling.
References:
Samkian, Adrian E.;Sercel, Zachary P.;Virgil, Scott C.;Stoltz, Brian M. [Tetrahedron Letters,2022,vol. 89,art. no. 153496] Location in patent:supporting information
![[1S-(1alpha,3beta,5beta,7alpha)]-3,8,8-trimethyl-4-oxatricyclo[5.1.0.03,5]octane](/CAS/GIF/936-91-4.gif)
936-91-4
1 suppliers
inquiry
![[1R-(1alpha,3alpha,4beta,6alpha)]-4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol](/CAS/GIF/4017-88-3.gif)
4017-88-3
2 suppliers
inquiry
![Ethanone, 1-[(1R,6S)-4,7,7-trimethylbicyclo[4.1.0]hept-4-en-3-yl]-](/CAS/20210305/GIF/620944-25-4.gif)
620944-25-4
0 suppliers
inquiry
![[1R-(1alpha,3alpha,4beta,6alpha)]-4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol](/CAS/GIF/4017-88-3.gif)
4017-88-3
2 suppliers
inquiry
![Bicyclo[4.1.0]hept-4-en-3-ol, 4,7,7-trimethyl-, (1R,3R,6S)-](/CAS/20200401/GIF/4017-82-7.gif)
4017-82-7
0 suppliers
inquiry
![[1R-(1alpha,3alpha,4beta,6alpha)]-4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol](/CAS/GIF/4017-88-3.gif)
4017-88-3
2 suppliers
inquiry
![[1R-(1alpha,3alpha,4alpha,6alpha)]-4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol](/CAS/GIF/4017-89-4.gif)