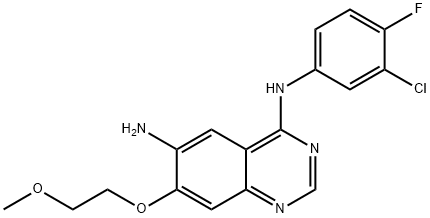
N4-(3-chloro-4-fluorophenyl)-7-(2-methoxyethoxy)quinazoline-4,6-diamine synthesis
- Product Name:N4-(3-chloro-4-fluorophenyl)-7-(2-methoxyethoxy)quinazoline-4,6-diamine
- CAS Number:402855-01-0
- Molecular formula:C17H16ClFN4O2
- Molecular Weight:362.79

402855-06-5
0 suppliers
inquiry

402855-01-0
17 suppliers
$439.00/1g
Yield:402855-01-0 93%
Reaction Conditions:
Stage #1: N-(3-chloro-4-fluorophenyl)-7-(2-methoxyethoxy)-6-nitroquinazoline-4-aminewith hydrogenchloride;iron in ethanol;water; for 2 h;Heating / reflux;
Stage #2: with sodium hydroxide in ethanol;water at 0; pH=11;
Steps:
28.28d
A mixture of 0306-68 (5.70 g, 14.5 mmol), ethanol (165 mL), water (43.5 mL) and hydrogen chloride (2.9 mL) was stirred to form a clear solution. The powder iron (16.24 g, 290.0 mmol) was added. The mixture was stirred at reflux for 2 hours. Cooled to room temperature, adjusted pH to 11 with 10% sodium hydroxide solution in ice-water bath and was filtered. The filtrate was concentrated to remove ethanol and extracted whit ethyl acetate (100 mL><2), The combined organic layer was washed with brine (30 mL><3) and dried over sodium sulfate, filtered and evaporated to give the title product 0308-68 as a yellow solid (4.92 g, 93%): LCMS: 363 [M+l]+.
References:
WO2008/33747,2008,A2 Location in patent:Page/Page column 136
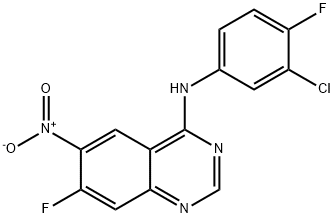
162012-67-1
340 suppliers
$8.00/1g

402855-01-0
17 suppliers
$439.00/1g
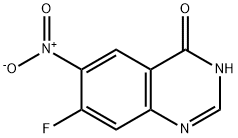
162012-69-3
372 suppliers
$6.00/250mg

402855-01-0
17 suppliers
$439.00/1g

162012-70-6
150 suppliers
$125.00/25mg

402855-01-0
17 suppliers
$439.00/1g
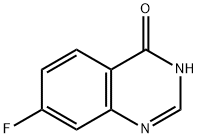
16499-57-3
236 suppliers
$6.00/250mg

402855-01-0
17 suppliers
$439.00/1g