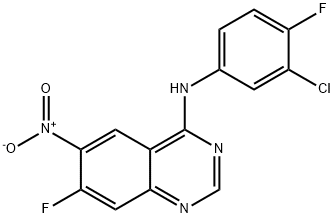
4-Quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitro- synthesis
- Product Name:4-Quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitro-
- CAS Number:162012-67-1
- Molecular formula:C14H7ClF2N4O2
- Molecular Weight:336.68
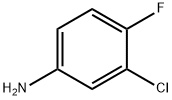
367-21-5
523 suppliers
$5.00/25g
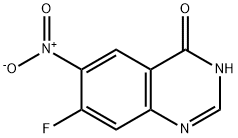
162012-69-3
367 suppliers
$6.00/250mg

162012-67-1
333 suppliers
$8.00/1g
Yield:162012-67-1 113 kg
Reaction Conditions:
Stage #1:7-fluoro-6-nitro-3H-quinazolin-4-one with triethylamine;trichlorophosphate in toluene at 80 - 90; for 6 h;Industrial scale;
Stage #2:3-chloro-4-fluorophenylamine in toluene at 40 - 50; for 1 h;Industrial scale;
Steps:
3 The third step: chlorinated 3,-chloro-4-fluoroaniline reaction:
90 kg of 7-fluoro-6-nitro-4-quinazolinone prepared in the second step was charged into 360 kg of toluene, and 72 kg of triethylamine was added.81 kg of phosphorus oxychloride was added dropwise at room temperature, and the temperature was raised to 80-90 ° C for 6 hours, and the temperature was lowered to 40-50 ° C.63 kg of 3-chloro-4-fluorine was added in portions, and the reaction was carried out at 40-50 ° C for 1 hour.The mixture was cooled to room temperature, centrifuged and dried to obtain 136 kg of a crude product. Purification of N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitro-4-quinazolinamine:The crude product obtained in the previous step was put into 816 kg of methanol, and triethylamine was added dropwise at room temperature to adjust the pH to 7-8.The mixture was heated to 50-60 ° C for 2 hours, cooled to room temperature, centrifuged and dried to obtain 113 kg (purity 99.6%).
References:
Nanjing Tian Yue Xing Biological Co., Ltd.;Wu Xueping;Chu Yijie CN109694357, 2019, A Location in patent:Paragraph 0022; 0033; 0034; 0035

162012-69-3
367 suppliers
$6.00/250mg

367-21-5
523 suppliers
$5.00/25g

162012-67-1
333 suppliers
$8.00/1g

367-21-5
523 suppliers
$5.00/25g

162012-70-6
147 suppliers
$125.00/25mg

162012-67-1
333 suppliers
$8.00/1g

446-32-2
448 suppliers
$6.00/5g

162012-67-1
333 suppliers
$8.00/1g
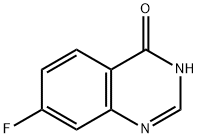
16499-57-3
239 suppliers
$6.00/250mg

162012-67-1
333 suppliers
$8.00/1g