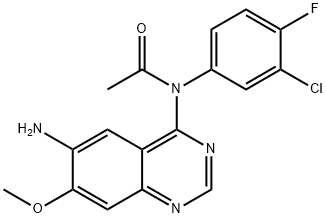
Acetamide, N-(6-amino-7-methoxy-4-quinazolinyl)-N-(3-chloro-4-fluorophenyl)- synthesis
- Product Name:Acetamide, N-(6-amino-7-methoxy-4-quinazolinyl)-N-(3-chloro-4-fluorophenyl)-
- CAS Number:869199-62-2
- Molecular formula:C17H14ClFN4O2
- Molecular Weight:360.77
869199-61-1
43 suppliers
inquiry

869199-62-2
20 suppliers
inquiry
Yield:869199-62-2 94.49%
Reaction Conditions:
with hydrogen;nickel in tetrahydrofuran at 60; under 3102.97 Torr; for 38 h;
Steps:
5
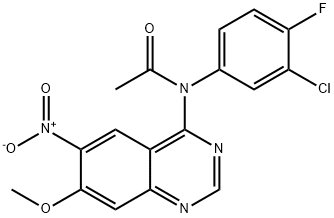
(3-Chloro4-fluoro-phenyl-(7-methoxy-6-nitro-quinazoline-4-yl)-amine (1) (1 7.26 g, 0.0495 mmol) was slurried in 350 ml acetic anhydride under nitrogen and warmed and maintained at 90° C. for 24 hrs and cooled gradually to RT. Pale colored slurry exists. Cooled to 0° C. for 1 hr. The solids were filtered and the flask and cake were washed with 2times50 ml IPA. The product, N-(3-Chloro-4-fluoro-phenyl)-N-(7-methoxy-6-nitro-quinazoline-4-yl)-acetamide (2), was dried in vacuum oven at 60° C. for 24 hrs. Mass: 17.97 g (92.4%). HPLC: 99.45%, rt=13.705 min. Raney Ni (5.0 g) was slurried in MeOH, followed by THF to remove water. N-(3-Chloro-4-fluoro-phenyl)-N-(7-methoxy-6-nitro-quinazoline-4-yl)-acetamide (2) (19.2 g, 49 mmol) was slurried in THF (500 ml) and charged to a reactor. The reaction was heated to 60° C. and pressurized with hydrogen to 60 psi. After almost 17 hrs, an additional 10.0 g of the catalyst was charged and the reaction was complete by 38 hrs. Filter reaction and wash with THF. The solids were concentrated on rotavap and the solvent was exchanged to hexanes. A pale yellow solid precipitated upon addition of hexanes. The solvent was removed under vacuum to distill any remaining THF. Filtered and washed with copious amounts of hexanes. The product, N-(6-Amino-7-methoxy-quinazolin-4-yl)-N-(3-chloro-4-fluoro-phenyl )-acetamide (3), was dried in vacuum oven at 70° C. for 24 hours. Mass is 16.75 g (94.49%). HPLC: tm (100%). Oxyalyl chloride was added to a solution of DMF (60 mg), 4-piperidin-1-yl-but-2-enoic acid in 40 ml DCM at room temperature and the reaction was stirred for one hour. The solvent was evaporated under vacuum and the resulting solid slurried in 150 ml DMAC. The N-(6-amino-7-methoxy-quinazolin-4-yl)-N-(3-chloro-4-fluoro-phenyl)-acetamide (3) was added to the reaction mixture as a solid. The reaction was completed after 45 minutes. The mixture was then added dropwise to 300 ml 2N NaOH and the aqueous layer was extracted into EtOAc. The combined organic layer was concentrated to 100 ml and stirred for 2 days at room temperature. 300 ml ethyl ether and 100 ml of 2N NaOH were added and the solid that precipitated out was collected by filtration. The final product was recrystallized from ethylene chloride to obtain 5.5 g pure product.
References:
US2005/250761,2005,A1 Location in patent:Page/Page column 18-20